Which reaction do you expect to have the smallest orientation factor? (a) H(g) + I(g) HI(g)
Question:
Which reaction do you expect to have the smallest orientation factor?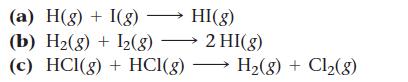
Transcribed Image Text:
(a) H(g) + I(g) — HI(g) (b) H₂(g) + 12(g) → 2 HI(g) (c) HCl(g) + HCI(g) H₂(g) + Cl₂(8)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
c Since the reactants in part a are atoms the orient...View the full answer

Answered By

Mustafa olang
Please accept my enthusiastic application to solutionInn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group. For example, I created songs to teach my three-year-old campers the camp rules, but I gave my college student daily quizzes to help her prepare for exams.
I am passionate about helping students improve in all academic subjects. I still remember my excitement when my calculus student received her first “A” on a quiz! I am confident that my passion and experience are the qualities you are looking for at solutionInn. Thank you so much for your time and consideration.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The entrance fee into Yellowstone National Park in northwestern Wyoming is "$50 for a private, noncommercial vehicle; $40 for a motorcycle; or $20 for each visitor 16 and older entering by foot,...
-
The entrance fee into Yellowstone National Park in northwestern Wyoming is $30 for a private, noncommercial vehicle; $25 for a motorcycle or a snowmobile; or $15 for each visitor 16 and older...
-
In 2019, the entrance fee into Yellowstone National Park in northwestern Wyoming was $35 for a private, noncommercial vehicle; $30 for a motorcycle or a snowmobile; and $15 for each visitor entering...
-
A $250 suit is on sale for $190, and a $90 pair of shoes is on sale for $65. Find the average percent decrease in price for the 2 items.
-
Data pertaining to job cost sheets for Knox Company are given in BE2-3 and BE2-4. Prepare the job cost sheets for each of the three jobs.
-
Assume that when a purchase of supplies of $3,100 for cash was recorded, both the debit and the credit were journalized and posted as $1,300. (a) Would this error cause the trial balance to be out of...
-
Refer to the information in Exercise 16-14. Prepare journal entries dated June 30 to record: (a) raw materials purchases, (b) direct materials usage, (c) indirect materials usage, (d) direct labor...
-
Capitalization of Interest On December 31, 2009, Hurston Inc. borrowed $3,000,000 at 12% payable annually to finance the construction of a new building. In 2010, the company made the following...
-
For this assignment, submit the code (SQL script or document), including structure from the Data Definition Language (DDL) and the Data Manipulation Language (DML). Use these SQL Statements as a...
-
The rate constant for a reaction at 25.0 C is 0.010 s -1 , and its activation energy is 35.8 kJ. Find the rate constant at 50.0 C. a) 0.021 s -1 b) 0.010 s -1 c) 0.0033 s -1 d) 0.031 s -1
-
Explain the difference between the rate law for a reaction and the integrated rate law for a reaction. What relationship does each kind of rate law express?
-
A chemical processing corporation is considering three methods to dispose of a non-hazardous chemical sludge: land application, fluidized-bed incineration, and private disposal contract. The...
-
Using company examples from different industries, examine the role of BHAGs in helping companies to produce far-reaching change.
-
Use the data given in question 34 to compute the change in real wages between 1986 and 1990. Are workers any better off? Question 34 Suppose you are given the following information about wages and...
-
Why is an understanding of the purpose of corporations helpful for understanding corporate governance?
-
Why does it work?
-
According to Larry Greiner, what is the purpose of the different evolutionary and revolutionary phases an organization experiences, as it grows and develops?
-
Woods Co. employed Brian Smith in 2013. Brian earned $5,400 per month and worked the entire year. Assume the Social Security tax rate is 6 percent for the first $110,000 of earnings and the Medicare...
-
What is the difference between the straight-line method of depreciation and the written down value method? Which method is more appropriate for reporting earnings?
-
With the previous problem in mind prove that dn(v) n(v) + v dv ng
-
With the previous problem in mind show that Data from Prob. 32 With the previous problem in mind prove that dn - g dn(v) n(v) + v dv ng
-
A well-known Optics book gives the equation Could this possibly be correct? Explain. [Check the units.] c dn = vl 1 1 dn do Vg n dk n? dk dk
-
6) For the geared system shown below, where ma is the actuation torque, and m, is the load torque. Formulate the mathematical model when known are: N = 24, N = 30, N3 = 36, N4 = 42, J = 4 10-5 kg-m,...
-
Assuming the gear train shown below is a lossless system, the input rotational velocity is Win =11 revolutions per minute (RPM). Given that there are 17 teeth on gears 2, 4, and 6 and 29 teeth on...
-
Determine the tension in cable CB and cable AC with alpha = 20 00 1200 lb

Study smarter with the SolutionInn App


