Without calculating E cell , predict whether each of the redox reactions is spontaneous. If the reaction
Question:
Without calculating E°cell, predict whether each of the redox reactions is spontaneous.
If the reaction is spontaneous as written, make a sketch of the electrochemical cell in which the reaction could occur. If the reaction is not spontaneous as written, write an equation for the spontaneous direction in which the reaction would occur and sketch the electrochemical cell in which the spontaneous reaction would occur. In your sketches, make sure to label the anode (which should be drawn on the left), the cathode, and the direction of electron flow.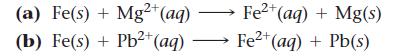
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





