Define the terms in Raoults law. Fig. 10.9 illustrates the net transfer of water molecules from pure
Question:
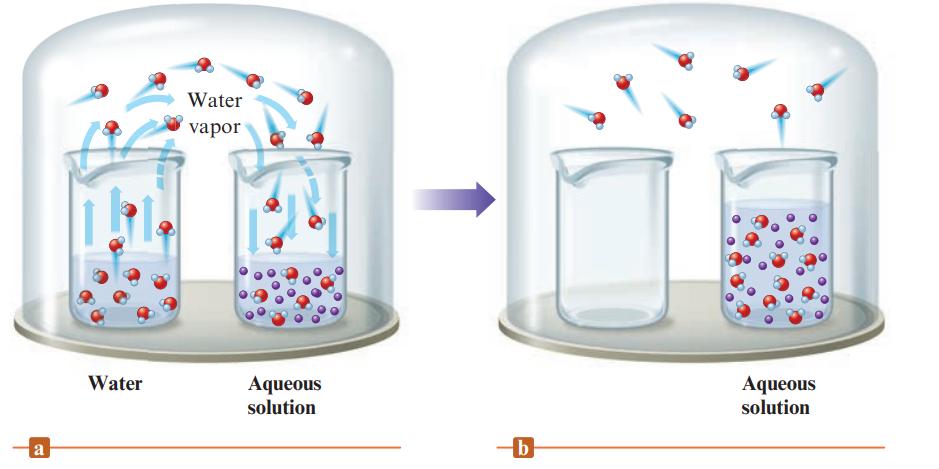
Define the terms in Raoult’s law. Fig. 10.9 illustrates the net transfer of water molecules from pure water to an aqueous solution of a nonvolatile solute. Explain why eventually all of the water from the beaker of pure water will transfer to the aqueous solution. If the experiment illustrated in Fig. 10.9 was performed using a volatile solute, what would happen? How do you calculate the total vapor pressure when both the solute and solvent are volatile?
Fig. 10.9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:





