The blood alcohol (C 2 H 5 OH) level can be determined by titrating a sample of
Question:
The blood alcohol (C2H5OH) level can be determined by titrating a sample of blood plasma with an acidic potassium dichromate solution, resulting in the production of Cr3+(aq) and carbon dioxide. The reaction can be monitored because the dichromate ion (Cr2O72-) is orange in solution, and the Cr3+ ion is green. The balanced equation is
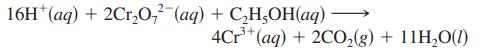
This reaction is an oxidation–reduction reaction. What species is reduced, and what species is oxidized? How many electrons are transferred in the balanced equation above?
Transcribed Image Text:
16H* (aq) + 2Cr₂O72 (aq) + C₂H₂OH(aq) - 4Cr³+ (aq) + 2CO₂(g) + 11H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
In the given chemical equation 16Haq 2CrOaq CHOHaq 4Craq 2COg 11HOl The species that gets reduced is ...View the full answer

Answered By

Shubhradeep Maity
I am an experienced and talented freelance writer passionate about creating high-quality content. I have over five years of experience working in the field and have collaborated with several renowned companies and clients in the SaaS industry.
At Herman LLC, an online collective of writers, I generated 1,000+ views on my content and created journal content for 100+ clients on finance topics. My efforts led to a 60% increase in customer engagement for finance clients through revamping website pages and email interaction.
Previously, at Gerhold, a data management platform using blockchain, I wrote and published over 50 articles on topics such as Business Finance, Scalability, and Financial Security. I managed four writing projects concurrently and increased the average salary per page from $4 to $7 in three months.
In my previous role at Bernier, I created content for 40+ clients within the finance industry, increasing sales by up to 40%.
I am an accomplished writer with a track record of delivering high-quality content on time and within budget. I am dedicated to helping my clients achieve their goals and providing exceptional results.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Explain why statisticians think the sample variance is a biased estimator of the population variance and how it could be corrected.
-
When markets suffer from imperfect information, it can decrease demand. (a) Why do consumers have a lower willingness to pay when they do not have enough information? (b) State one way that firms can...
-
1) Consider a wave packet for which A(K) = {0 (N, Obtain (x, 0), Ak, Ax and show that Ak^x is independent of k - 2K x 2K elsewhere
-
Suppose the graph represents the sales of goods of a company from the year 2000 to 2008. a) Find the average rate of change from x = 1 to x = 4 and interpret. b) Find the average rate of change from...
-
Accounting professionals that work for private companies often obtain the Certified Management Accountant (CMA) designation to indicate their proficiency in several business areas in addition to...
-
For Concept 1, the statement is correct regarding the effects on: A. the real tax savings from depreciation, but incorrect regarding the real after-tax interest expense. B. both the real tax savings...
-
Discuss the themes, theory, and/or phenomenon that would be anticipated to emerge as a result of the examination. Develop a hypothetical research scenario that would necessitate the use of the Action...
-
Kirsoff Company makes eBook readers. The company had the following amounts at the beginning of 2011: Cash, $660,000; Raw Materials Inventory, $51,000; Work in Process Inventory, $18,000; Finished...
-
1. Prove: 1 + 2 + 3 + - + n = (n + 1) using lattice paths. 1+2+3++n= n+ 2 2. Solve a = 2n+1 - 1 with a = 1.
-
Your first assignment in your new position as assistant financial analyst at Caledonia Products is to evaluate two new capital- budgeting proposals. Because this is your first assignment, you have...
-
The vanadium in a sample of ore is converted to VO 2+ . The VO 2+ ion is subsequently titrated with MnO 4 - in acidic solution to form V(OH) 4 + and manganese(II) ion. The unbalanced titration...
-
Consider the reaction between oxygen (O 2 ) gas and magnesium metal to form magnesium oxide. Using oxidation states, how many electrons would each oxygen atom gain, and how many electrons would each...
-
A Ford Focus car and a Freightliner Cascadia truck leave an intersection at the same time. The Focus heads east at an average speed of 60 miles per hour, while the Cascadia heads south at an average...
-
Two point charges, +4 microC and -1 microC, lie on the x-axis and are separated by a distance of 1 meter as shown in the Figure. If the +4 microC lies at the origin, at what point (or points) on the...
-
Stevie owns two personal residences that satisfy the two-year ownership and use test with respect to the five-year window. The Orchard Street residence has a cost basis of $220,000, and the Main...
-
MTN was able to raise only GH1.15billion out of the expected GH3.48billion from its Initial Public Offering which lasted from May 29, 2018 to July 31, 2018. Even though the share sale exceeded the...
-
Slinky made personal superannuation contributions of $23,000 throughout the year. He was also employed by another company to provide business consulting services to archery-specific businesses on...
-
Georgia leased a car as of March 1, 2022, at a cost of $750 a month, including GST. At the time that she leased the car, the manufacturer's list price on the car was $44,625 including GST. During...
-
The accounts and balances that follow are from Hastings Corporations records on December 31, 2014. Preferred Stock, $100 par value, 9 percent cumulative, 10,000 shares authorized, 6,000 shares issued...
-
(a) Explain why the concentration of dissolved oxygen in freshwater is an important indicator of the quality of the water. (b) How is the solubility of oxygen in water affected by increasing...
-
Why is it necessary to functionalize CdSe quantum dots with groups such as organic acids to make them useful in bioanalytical applications?
-
Why must the amplitudes of the first derivatives of the energy eigenfunctions in the finite depth box and in the adjoining barrier regions have the same value at the boundary?
-
Why must the amplitudes of the energy eigenfunctions in the finite depth box and in the adjoining barrier regions have the same value at the boundary?
-
Video Post 1: Define the different buying situations and what that means to a negotiation. Discuss what the difference is between a feature, advantage and benefit are to the customer. Discuss the...
-
1. What is your decision-making style? 2. Do the results match what you believe about your personality? 3. What should you do to improve your decision-making abilities?
-
What is the revenue growth trend of the potential partner in the last three years? How do the potential partner's financial ratios compare to industry benchmarks? Are there any outstanding tax...

Study smarter with the SolutionInn App


