Write the equilibrium expression (K) for each of the following gas-phase reactions. a. N(g) + O(g)=2NO(g) b.
Question:
Write the equilibrium expression (K) for each of the following gas-phase reactions.
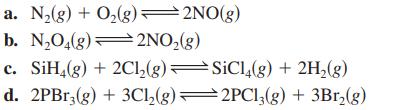
Transcribed Image Text:
a. N₂(g) + O₂(g)=2NO(g) b. N₂O₂(g)2NO₂(g) c. SiH4(g) + 2Cl₂(g) d. 2PBr3(g) + 3Cl₂(g) SiCl4(g) + 2H₂(g) 2PC13(g) + 3Br₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry An Atoms First Approach
ISBN: 9781305079243
2nd Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl
Question Posted:
Students also viewed these Sciences questions
-
Consider a gas phase reaction where N2O4 (g) 2NO2 (g) at 25C. The equilibrium constant (Kp) for the reaction is 0.25 atm. a) Write the equilibrium expression for Kp. b) Calculate the partial...
-
A stirred tank with volume V t? (L) is charged with V 1 (L) of a liquid. B. The space above the liquid (volume V g = V t ? V 1 ) is filled with a pure gas, A, at an initial pressure P 0 (atm). The...
-
Give the expression for K for each of the following reactions. a. b. c. CaCO (s)CaO(s) CO2(g) Pbl2(s) H (a) HCO (aq)H2O) CO2(g)
-
A slipper-pad bearing (Fig. P1023) is often encountered in lubrication problems. Oil flows between two blocks; the upper one is stationary, and the lower one is moving in this case. The drawing is...
-
What If the Facts Were Different? Suppose that shortly before Pallisters death, she had asked James to tear up her will, and he had done it. Would the result have been different? Explain.
-
Determining Diet One method for determining the amount of com in early Native American diets is the stable isotope ratio analysis (SIRA) technique. As com photosynthesizes, it concentrates the...
-
When conducting an incremental analysis, what step must always be taken immediately prior to beginning the pairwise comparisons? a. Order the alternatives from highest to lowest initial investment b....
-
Suppose the amounts presented here are basic financial information (in millions) from the 2014 annual reports of Nike and adidas. Instructions Calculate the accounts receivable turnover and average...
-
A monopoly water slide has demand of p(Q) = a - bQ, where p is the price per slide ("ride"), Q is the quantity of slides demanded, and a, b > 0. Fixed costs are F = 0 and constant marginal costs are...
-
The second column in Table 13.1 shows the monthly return on the British FTSE 100 index from January 2015 through July 2017. The remaining columns show returns on the stocks of two firms?Executive...
-
Consider the following reaction at a certain temperature: An equilibrium mixture contains 1.0 mole of Fe, 1.0 10 -3 mole of O 2 , and 2.0 moles of Fe 2 O 3 all in a 2.0-L container. Calculate the...
-
For a typical equilibrium problem, the value of K and the initial reaction conditions are given for a specific reaction, and you are asked to calculate the equilibrium concentrations. Many of these...
-
One principle of fraud is that fraud is hidden. What are the implications of this principle to the fraud investigator?
-
Choose the correct sentence. options: The elections will be held on Tuesday November 6, 2018, and the polls will be kept open until 8:00 p.m. The elections will be held on Tuesday, November 6, 2018,...
-
Costco (https://www.youtube.com/watch?v=-pXmKb6V_O4) Hubstop (https://www.youtube.com/watch?v=3iKPrDFruoU) Based on what you have learned from viewing the two videos, compare the VALUES of HUBSTOP...
-
What type of funds aim for safety of principal and income but are subject to capital gains and losses?
-
Instructions: Under settings, click "Price Ceiling" to select it. When it is selected, it will be highlighted in dark blue. a. What is the equilibrium price and quantity in the absence of a price...
-
To add to your understanding, it's important to note that auditors also need to consider the risk associated with different sampling units when determining the sample size. Higher-risk items or...
-
Bill Joyner is evaluating a new ticketing system for his theater. The system will cost $225,000 and will save the theater $57,275 in annual cash operating costs. Bill expects the new system to last...
-
What do you think?
-
Assign a name for each of the following compounds: (a) (b) (c) (d) (e) (f) `NH2 NH2
-
Draw all constitutional isomers with molecular formula C 4 H 11 N, and provide a name for each isomer.
-
Draw all tertiary amines with molecular formula C 5 H 13 N, and provide a name for each isomer. Are any of these compounds chiral?
-
Policymakers are contemplating undertaking either an increase in government spending or an increase in the money supply. Either policy is forecast to have the same impact on income in the short run....
-
What is your current philosophy for driving change in the health care industry? Speak from an dialysis experience in the health care industry and insight from the MHA program. Include an example of...
-
Prepare a physical unit flow reconciliation with the following information. Blending Process Units of Product Beginning work in process inventory 168,000 Units started this period 355,000 Units...

Study smarter with the SolutionInn App


