A 2011 article in Science magazine stated that Public discussion of climate change . . . is
Question:
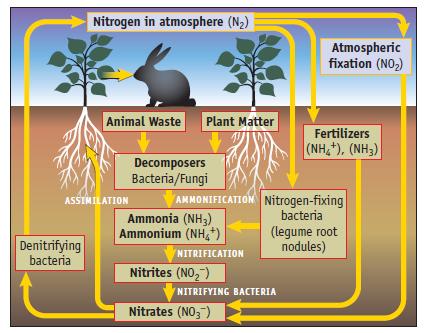
A 2011 article in Science magazine stated that “Public discussion of climate change . . . is just beginning to reflect an awareness of the important role played by the global nitrogen cycle.” (Figure 20.1.) As part of this cycle it was recently found that nitrite ions in soil can produce nitrous acid, HONO. [M. Kulmala and T. Petaja, Science, Vol. 333, pp. 1586–1587, 2011.]
(a) Draw the Lewis electron dot structure for HONO and indicate the electron-pair geometry around the O and N atoms.
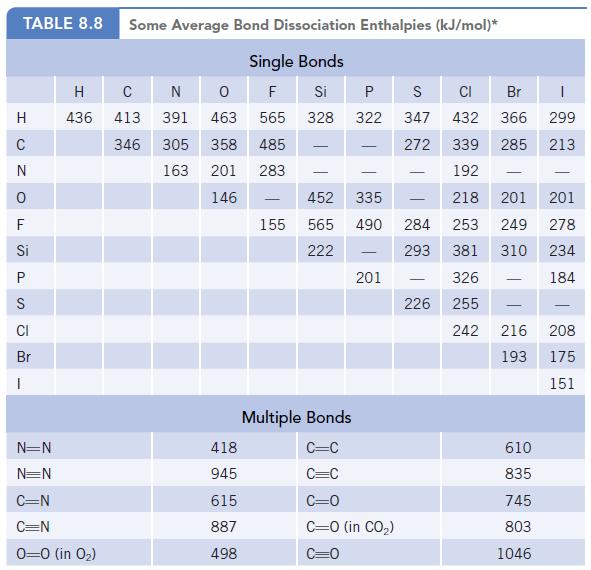
(b) HONO is a source of hydroxyl radicals, ·OH, in the atmosphere because enough energy is supplied by sunlight to break the N—O bond. Use the bond dissociation enthalpy of the N—O bond (Table 8.8) to calculate the wavelength of light that could cause HONO to dissociate to the radicals ·NO and ·OH.
Data given in Figure 20.1
 Data given in Table 8.8
Data given in Table 8.8
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel




