A 4.000-g sample containing KCl and KClO 4 was dissolved in sufficient water to give 250.00 mL
Question:
A 4.000-g sample containing KCl and KClO4 was dissolved in sufficient water to give 250.00 mL of solution. A 50.00-mL portion of the solution required 41.00 mL of 0.0750 M AgNO3 in a Mohr titration. Next, a 25.00-mL portion of the original solution was treated with V2(SO4)3 to reduce the perchlorate ion to chloride,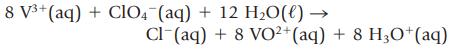
and the resulting solution was titrated with AgNO3. This titration required 38.12 mL of 0.0750 M AgNO3. What is the mass percent of KCl and KClO4 in the mixture?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





