A noncarbonated soft drink contains an unknown amount of citric acid, H 3 C 6 H 5
Question:
A noncarbonated soft drink contains an unknown amount of citric acid, H3C6H5O7. If 100. mL of the soft drink requires 33.51 mL of 0.0102 M NaOH to neutralize the citric acid completely, what mass of citric acid does the soft drink contain per 100. mL? The reaction of citric acid and NaOH is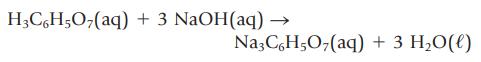
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





