About 5 billion kilograms of benzene, C 6 H 6 , are made each year. Benzene is
Question:
About 5 billion kilograms of benzene, C6H6, are made each year. Benzene is used as a starting material for many other compounds and as a solvent (although it is also a carcinogen, and its use is restricted). One compound that can be made from benzene is cyclohexane, C6H12.
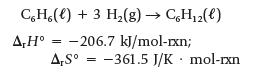
Is this reaction predicted to be product-favored at equilibrium at 25°C? Is the reaction enthalpy- or entropy-driven?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





