Balance the following equations: (a) For the reaction to produce superphosphate fertilizer (b) For the reaction to
Question:
Balance the following equations:
(a) For the reaction to produce “superphosphate” fertilizer
![]()
(b) For the reaction to produce diborane, B2H6
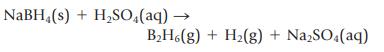
(c) For the reaction to produce tungsten metal from tungsten(VI) oxide ![]() (d) For the decomposition of ammonium dichromate
(d) For the decomposition of ammonium dichromate
![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





