Calcium carbide, CaC 2 , is manufactured by the reaction of CaO with carbon at a high
Question:
Calcium carbide, CaC2, is manufactured by the reaction of CaO with carbon at a high temperature. (Calcium carbide is then used to make acetylene.)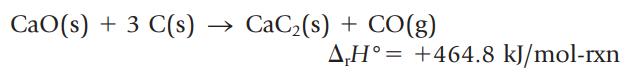
Is this reaction endothermic or exothermic? What is the enthalpy change if 10.0 g of CaO is allowed to react with an excess of carbon?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





