Calculate the pH of a 0.12 M aqueous solution of the base aniline, C 6 H 5
Question:
Calculate the pH of a 0.12 M aqueous solution of the base aniline, C6H5NH2 (Kb = 4.0 × 10−10).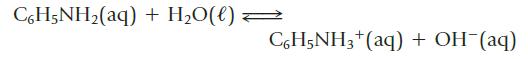
Transcribed Image Text:
CoH5NHz(aq) +H,O(l)< C6H5NH3+ (aq) + OH-(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
To calculate the pH of a 012 M aqueous solution of the base aniline C6H5NH2 you can use the concept ...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Lets take a look at the extensive career of Helen Frankenthaler (1928-2011), who found a way to incorporate all of the innovative techniques artists were experimenting with starting in 1945. Her...
-
Calculate the pH of a 0.15 M aqueous solution of aluminum chloride, AlCl3. The acid ionization of hydrated aluminum ion is and Ka is 1.4 10-5.
-
Calculate the pH of a 0.15 M aqueous solution of zinc chloride, ZnCl2. The acid ionization of hydrated zinc ion is and Ka is 2.5 10-10.
-
Sketch the six graphs of the x- and y-components of position, velocity, and acceleration versus time for projectile motion with x 0 = y 0 = 0 and 0 < 0 < 90.
-
What are the advantages and disadvantages of having an employer draw attention to situations where employees use some of their work time to do non-job-related activities? Does labeling this employee...
-
A beam of unpolarized light carries 2000 W/m 2 down onto an airplastic interface. It is found that of the light reflected at the interface 300 W/m 2 is polarized with its E-field perpendicular to the...
-
Use the data given in Problem 12.18 and plot the dimensionless coefficients \(C_{H}, C_{\mathscr{P}}, \eta\) versus \(C_{Q}\) for this pump. Calculate a meaningful value of specific speed, discuss...
-
For KnightRider Company, the predetermined overhead rate is 150% of direct labor cost. During the month, KnightRider incurred $100,000 of factory labor costs, of which $85,000 is direct labor and...
-
What are the key considerations in integrating hazard analysis with system safety engineering and risk management frameworks, such as ISO 31000 and ANSI/ASSP Z590.3? Discuss the synergies and...
-
Suppose you have $5,000 in savings when the price level index is at 100. (a) If inflation pushes the price level up by 10 percent, what will be the real value of your savings? (b) What is the real...
-
Hemoglobin (Hb) can form a complex with both O 2 and CO. For the reaction at body temperature, K is about 200. If the ratio [HbCO]/[HbO 2 ] comes close to 1, death is probable. What partial pressure...
-
Construct the possible Eigenvectors for the degenerate modes in the case of the three coupled pendula by requiring a11 = 2a21. Interpret this situation physically.
-
Image intensifiers used in nightvision devices create a bright image from dim light by letting the light first fall on a photocathode. Electrons emitted by the photoelectric effect are accelerated...
-
When we say that a photon is a "quantum of light," what does that mean? What is quantized?
-
An investigator is measuring the current in a photoelectric effect experiment. The cathode is illuminated by light of a single wavelength. What happens to the current if the wavelength of the light...
-
In a photoelectric effect experiment, the intensity of the light is increased while the frequency, which is above the threshold frequency, is held constant. As a result, A. There are more electrons....
-
A gold cathode is illuminated with light of wavelength \(250 \mathrm{~nm}\). It is found that the current is zero when \(\Delta V=1.0 \mathrm{~V}\). Would the current change if a. The light intensity...
-
Based on the figures in practice problems 17 and 18, how much money did the shareholders actually invest in the firm (i.e., what is the value of the capital stock)?
-
Find the work done in pumping all the oil (density S = 50 pounds per cubic foot) over the edge of a cylindrical tank that stands on one of its bases. Assume that the radius of the base is 4 feet, the...
-
Determine the support reactions at and . Take E = 200 MPa, I = 300(10 6 ) mm 4 , A = 21(10 3 ) mm 2 for each member. - 5 m 300 kN m, 3 9. (2, 4 m 2
-
Determine the structure stiffness matrix K for the frame. Take E = GPa, I = 350(10 6 ) mm 4 , A = 15(10 3 ) mm 2 for each member. Joints at and are pins. 2 60 kN 3 |1 2) 2 m - - 2 m 2 4 m
-
Determine the support reactions at pins and . Take E = 200 GPa, I = 350(10 6 ) mm 4 , A = 15(10 3 ) mm 2 for each member. 2 60 kN 3 - 2 m 2 m 4 m 3) 9
-
During the year, TRC Corporation has the following inventory transactions. Date January 1 Transaction Beginning inventory April 7 Purchase July 16 Purchase October 6 Purchase Number of Unit Total...
-
Global Industrial intends to execute a marketing program half a year from now that requires a $ 5 0 0 , 0 0 0 investment. In preparation, the company plans to invest some of its available funds into...
-
When using the four-step process for organizing creative thinking, which step involves ideas beginning to flow?

Study smarter with the SolutionInn App


