CaTiO 3 , a perovskite, has the structure below. (a) If the density of the solid is
Question:
CaTiO3, a perovskite, has the structure below.
(a) If the density of the solid is 4.10 g/cm3, what is the length of a side of the unit cell?
(b) Calculate the radius of the Ti4+ ion in the center of the unit cell. How well does your calculation agree with a literature value of 75 pm?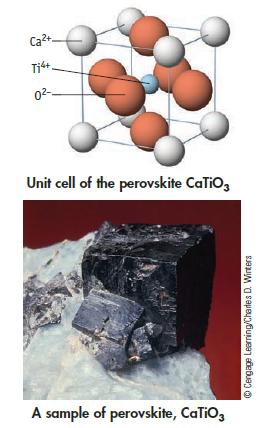
Transcribed Image Text:
Ca²+ Ti4+. 0²- Unit cell of the perovskite CaTiO3 A sample of perovskite, CaTiO3 ⒸCengage Learning/Charles D. Winters
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
a To find the length of a side of the unit cell for CaTiO3 we need to calculate the volume of the un...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
KYC's stock price can go up by 15 percent every year, or down by 10 percent. Both outcomes are equally likely. The risk free rate is 5 percent, and the current stock price of KYC is 100. (a) Price a...
-
9.3.1 Which of the following statements are true concerning the mean of the differences between two dependent samples (matched pairs)? Select all that apply. A. The requirement of a simple random...
-
Critique Pepsis handling of Baesa. Could it have prevented the South American disaster? If so, how?
-
Meredith, who is single, would like to contribute $5,500 to her Roth IRA. What is the maximum amount that Meredith can contribute if her AGI is $117,000?
-
Do analytical efforts, big data examinations, and textual analyses impact compliance and fraud deterrence? Provide an explanation for your opinion.
-
The adjusted trial balance of Karise Repairs on December 31, 2013, follows. Required 1. Prepare an income statement and a statement of owners equity for the year 2013, and a classified balance sheet...
-
Daniela built a raised garden bed that is 15 feet long, 6 feet wide, and 2.5 feet tall. She plans to fill the garden bed with premium soil that costs $15.96 per cubic yard. Recall that 1 yard = 3...
-
Molecular solids, network solids, and amorphous solids all contain atoms that are joined together by covalent bonds. However, these classes of compounds are very different in overall structure, and...
-
Calculate the lattice energy of CaCl 2 using a Born- Haber cycle and data from Appendices F and L and Table 7.5. Data given in Table 7.5 TABLE 7.5 2nd Period 1st 2nd 3rd 3rd Period 1st 2nd 3rd 4th...
-
Refer to Exercise 8.S.6. Analyze these data using a Wilcoxon signed-rank test. Exercise 8.S.6. Biologists noticed that some stream fishes are most often found in pools, which are deep, slow-moving...
-
Consider a firm with Cobb-Douglas production function F(K,L) = 4K2L3, where K sem fors oljart opetova od 2000 i od bus denotes units of capital and L represents units of labor. Assume that the firm...
-
How can inventory management improve the operational performance of M&S (Marks and Spencer)?
-
What is the purpose of a front desk information directory? What sort of information might such a directory contain? How could Hotel Alore benefit from the use of an Information Directory?
-
Consider a particle V0 X=0 incident. V=V II m V=O upon a potential barrier X=L (a) Given a - particle incident from the left write expressions for the wave function in the three regions for the case E
-
6) Discuss whether an online platform i.e., google will be held responsible for the defamatory content of third-party reviews and other posts or for hyperlinks?
-
The following information is available for Lotus Lake, Inc., as of May 31, 2011: Cash on the books as of May 31 amounted to $43,784.16. Cash on the bank statement for the same date was $53,451.46. ...
-
Find the numerical value of each expression. (a) sech 0 (b) cosh -1 1
-
The hanger is supported using the rectangular pin. Determine the required thickness t of the hanger, and dimensions a and b if the suspended load is P = 60 kN. The allowable tensile stress is (Ï...
-
The rods AB and CD are made of steel. Determine their smallest diameter so that they can support the dead loads shown. The beam is assumed to be pin connected at A and C. Use the LRFD method, where...
-
The aluminum bracket A is used to support the centrally applied load of 8 kip. If it has a thickness of 0.5 in., determine the smallest height h in order to prevent a shear failure. The failure shear...
-
Pinehurst Company was formed in Year 1 and experienced the following accounting events during the year: 1. Issued common stock for $18,400 cash. 2. Earned cash revenue of $26,100. 3. Paid cash...
-
Use the below table to answer the following questions. Selling Price $43.00 = Sales Volume Variable 2,200 3,200 Fixed Cost Cost 4,200 Profitability 5,200 6,200 $47,200 15 $14,400 $42,400 $70,400...
-
Simon Company's year-end balance sheets follow. At December 31 Assets Current Year 1 Year Ago 2 Years Ago Cash Accounts receivable, net $ 34,375 99,630 $ 40,993 69,607 Merchandise inventory Prepaid...

Study smarter with the SolutionInn App


