Chemists sometimes carry out reactions in liquid ammonia as a solvent. With adequate safety protection these reactions
Question:
Chemists sometimes carry out reactions in liquid ammonia as a solvent. With adequate safety protection these reactions can be done at temperatures above ammonia’s boiling point in a sealed, thick walled glass tube. If the reaction is being carried out at 20°C, what is the pressure of ammonia inside the tube? (Use data from the previous question to answer this question.)
Data given in Previous Question
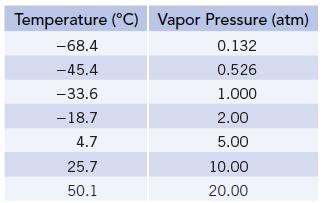
Vapor pressures of NH3(ℓ) at several temperatures are given in the table below. Use this information to calculate the enthalpy of vaporization of ammonia.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





