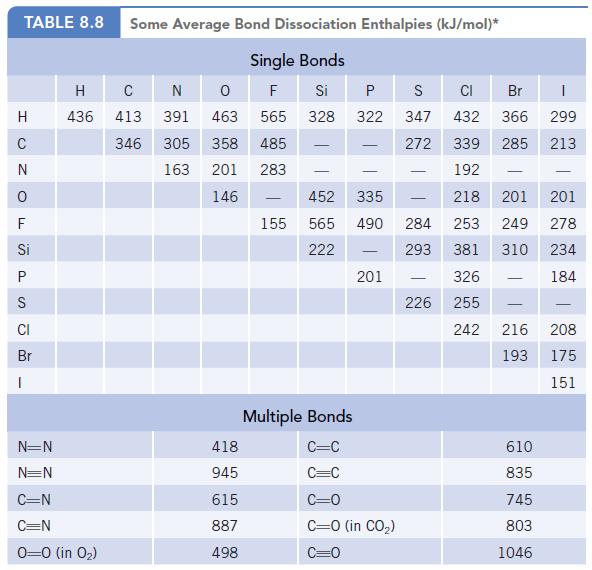
Chlorine atoms are formed by photochemical reactions of chlorofluorocarbons in the upper atmosphere. Using the average bond
Question:
Chlorine atoms are formed by photochemical reactions of chlorofluorocarbons in the upper atmosphere. Using the average bond energy of the C—Cl bond in Table 8.8, calculate the wavelength of radiation with sufficient energy to break the C—Cl bond. In what region of the electromagnetic spectrum does this fall?
Data given in Table 8.8
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





