Draw resonance structures for the SO 2 molecule, and determine the formal charges on the S and
Question:
Draw resonance structures for the SO2 molecule, and determine the formal charges on the S and O atoms. Are the S—O bonds polar, and is the molecule as a whole polar? If so, what is the direction of the net dipole in SO2? Is your prediction confirmed by the electrostatic potential surface? Explain briefly.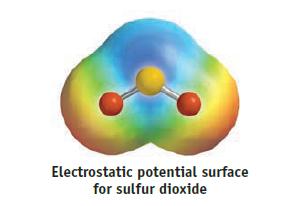
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





