Enthalpy changes for the following reactions can be determined experimentally: Use these values to determine the enthalpy
Question:
Enthalpy changes for the following reactions can be determined experimentally: 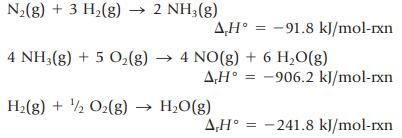
Use these values to determine the enthalpy change for the formation of NO(g) from the elements (an enthalpy change that cannot be measured directly because the reaction is reactant-favored). ![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





