For the reaction TiCl 2 (s) + Cl 2 (g) TiCl 4 (), r G
Question:
For the reaction TiCl2(s) + Cl2(g) → TiCl4(ℓ),
ΔrG° = −272.8 kJ/mol-rxn. Using this value and other data available in Appendix L, calculate the value of Δf G° for TiCl2(s).
Data given in Appendix L
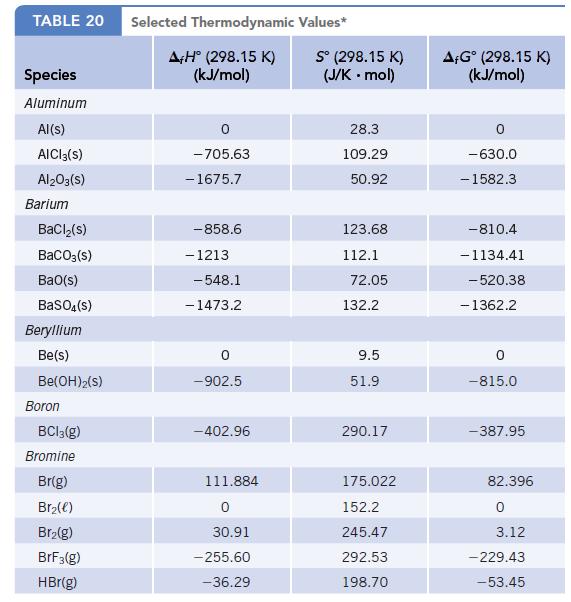
Transcribed Image Text:
TABLE 20 Species Aluminum Al(s) AICI 3(S) Al2O3(S) Barium BaCl₂(s) BaCO3(s) BaO(s) BaSO4(s) Beryllium Be(s) Be(OH)2(S) Boron BC13(g) Bromine Br(g) Br₂(e) Br₂(g) BrF3(g) HBr(g) Selected Thermodynamic A+H° (298.15 K) (kJ/mol) -705.63 - 1675.7 -858.6 - 1213 -548.1 -1473.2 0 -902.5 -402.96 111.884 0 30.91 -255.60 -36.29 Values* Sº (298.15 K) (J/K.mol) 28.3 109.29 50.92 123.68 112.1 72.05 132.2 9.5 51.9 290.17 175.022 152.2 245.47 292.53 198.70 AFGᵒ (298.15 K) (kJ/mol) -630.0 - 1582.3 -810.4 -1134.41 -520.38 - 1362.2 0 -815.0 -387.95 82.396 0 3.12 -229.43 -53.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate the standard Gibbs free energy change for the formation of TiCl2s from its elements at ...View the full answer

Answered By

Jinah Patricia Padilla
Had an experience as an external auditor in Ernst & Young Philippines and currently a Corporate Accountant in a consultancy company providing manpower to a 5-star hotel in Makati, Philippines, Makati Diamond Residences
5.00+
120+ Reviews
150+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
For the reaction BaCO 3 (s) BaO(s) + CO 2 (g), r G = +219.7 kJ/mol-rxn. Using this value and other data available in Appendix L, calculate the value of f G for BaCO 3 (s). Data given in Appendix L...
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
The Regal Cycle Company manufactures three types of bicycles-a dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Sales Variable manufacturing and...
-
What advice would you give to Michelle about her selection of participants for her focus groups? Michelle had been a student union welfare officer on a years sabbatical from her undergraduate...
-
Because new businesses are resource constrained, they often make partnering an essential part of their business plans. As illustrated throughout this book, effective partnering can help a start-up in...
-
The pressure at which a pure substance changes its phase is known as the (a) Saturated pressure (b) Gauge pressure (c) Absolute pressure (d) None of these.
-
Reid Chocolates (see problem 9.6) is considering a third layout, as shown below. Evaluate its effectiveness in trip-distance feet. In problem 9.6, you have just been hired as the director of...
-
In the summer of 1976, a busload of children, together with their bus driver, Ed Ray, were kidnappedbus and allfrom a country road in Madera County in California. The abductors ditched the bus,...
-
Determine whether the reactions listed below are entropy-favored or disfavored under standard conditions. Predict how an increase in temperature will affect the value of r G. (a) N 2 (g) + 2 O 2 (g)...
-
Using values of f H and S, calculate the standard molar free energy of formation, f G, for each of the following: (a) Ca(OH) 2 (s) (b) Cl(g) (c) Na 2 CO 3 (s) Compare your calculated values of f G...
-
Sending employees and their families to live and work for several years and then re-integrating them on return is obviously expensive. Why do companies do it instead of employing local personnel?
-
Kingsport Containers Company makes a single product with wide seasonal variations in demand. The company uses a job-order costing system and computes plantwide predetermined overhead rates on a...
-
Miller Company manufactures a line of lightweight running shoes. CEO Richard Miller estimated that the company would incur $7,000,000 in manufacturing overhead during the coming year. Additionally,...
-
A composite made of aluminum matrix with steel fibers is designed for carrying electrical power. Also, the electrical resistivity of aluminum is 50 x10^-7 Ohm.cm and that of steel is 400x10^-7...
-
A bank is loaning $5,000 for three years at an interest rate of 7.5 percent. How much additional interest can the bank earn if it compounds interest continuously rather than annually?
-
The Equal Employment Opportunity Commission (EEOC) was established in 1965 and its purpose is to enforce "federal laws that make it illegal to discriminate against a job applicant or an employee...
-
1. What are the principal classes of capital assets associated with governmental activities that Vero Beach reports in the financial statements? 2. Some analysts use the accumulated depreciation...
-
You continue to work in the corporate office for a nationwide convenience store franchise that operates nearly 10,000 stores. The per- store daily customer count (i.e., the mean number of customers...
-
The Lyman series in the hydrogen atom corresponds to transitions that originate from the n = 1 level in absorption or that terminate in the n = 1 level for emission. Calculate the energy, frequency...
-
Calculate the wavelengths of the first three lines of the Lyman, Balmer, and Paschen series, and the series limit (the shortest wavelength) for each series.
-
How many ways are there to place three electrons into an f sub-shell? What is the ground-state term for the f 3 configuration, and how many states are associated with this term? See Problem P 22.36.
-
Marketing fixed costs were $200,000 more than planned but manufacturing and general overhead costs were $100,000 less than planned. What was the Fixed Cost Variance?
-
2. Forces and accelerations Consider the following situations. In each case, say whether or not a net force has been applied to the object and explain your answer in a sentence or two. (a) You see a...
-
S COL A parallel plate capacitor consists of two isolated circular plates with diameter d separated by a distance s, as shown above (s < < d). The charge density of each plate has magnitude , and the...
Statistical Methods For The Social Sciences 4th International Edition - ISBN: 013713150X - Free Book

Study smarter with the SolutionInn App


