If you have silverware in your home, you know it tarnishes easily. Tarnish is from the oxidation
Question:
If you have “silverware” in your home, you know it tarnishes easily. Tarnish is from the oxidation of silver in the presence of sulfur-containing compounds (in the atmosphere or in your food) to give black Ag2S. To remove the tarnish, you can warm the tarnished object with some aluminum foil in water with a small amount of baking soda. Silver sulfide reacts with aluminum to produce silver as well as aluminum oxide and hydrogen sulfide. ![]()
Hydrogen sulfide is foul smelling, but it is removed by reaction with the baking soda.
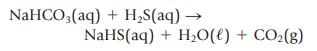
Classify the two reactions, and identify any acids, bases, oxidizing agents, or reducing agents.

Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





