Insoluble AgCl(s) precipitates when solutions of AgNO 3 (aq) and NaCl(aq) are mixed. To measure the energy
Question:
Insoluble AgCl(s) precipitates when solutions of AgNO3(aq) and NaCl(aq) are mixed.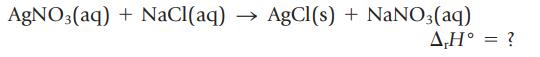
To measure the energy evolved in this reaction, 250. mL of 0.16 M AgNO3(aq) and 125 mL of 0.32 M NaCl(aq) are mixed in a coffee-cup calorimeter. The temperature of the mixture rises from 21.15°C to 22.90°C. Calculate the enthalpy change for the precipitation of AgCl(s), in kJ/mol. (Assume the density of the solution is 1.0 g/mL and its specific heat capacity is 4.2 J/g ∙ K.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





