Mass spectrometric analysis showed that there are four isotopes of an unknown element having the following masses
Question:
Mass spectrometric analysis showed that there are four isotopes of an unknown element having the following masses and abundances: 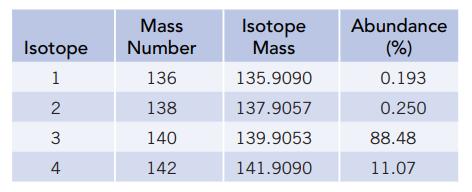
Three elements in the periodic table that have atomic weights near these values are lanthanum (La), atomic number 57, atomic weight 138.9055; cerium (Ce), atomic number 58, atomic weight 140.115; and praseodymium (Pr), atomic number 59, atomic weight 140.9076. Using the data above, calculate the atomic weight, and identify the element if possible.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





