Oxalic acid is a relatively weak diprotic acid. Calculate the equilibrium constant for the reaction shown below
Question:
Oxalic acid is a relatively weak diprotic acid. Calculate the equilibrium constant for the reaction shown below from Kal and Ka2. (See Appendix H for the required Ka values.)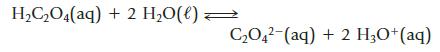
Data given in Appendix H
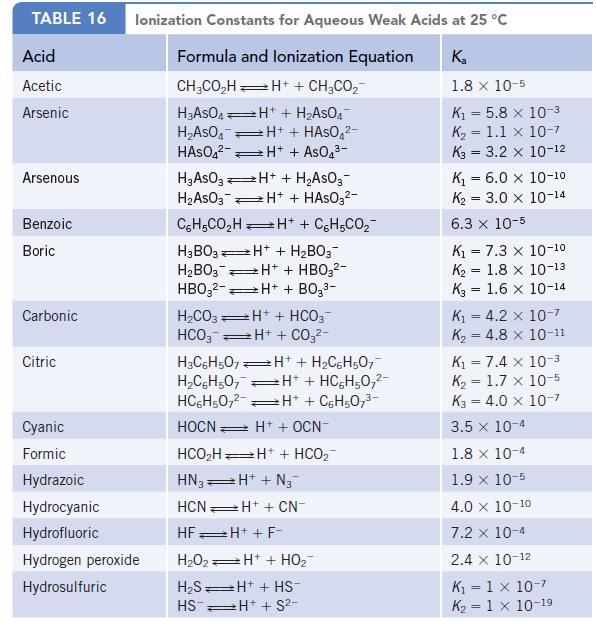
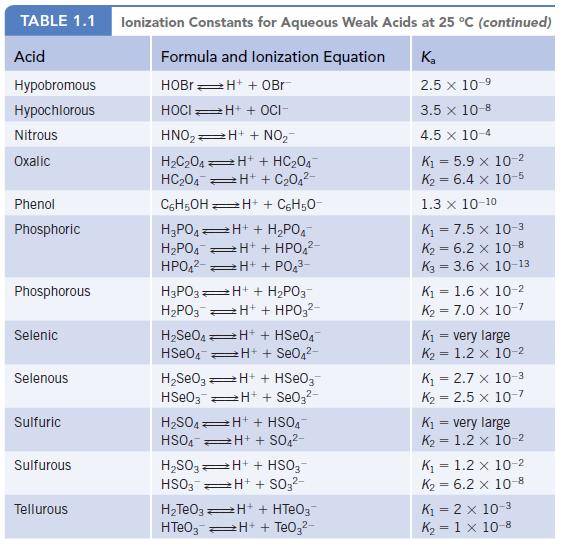
Transcribed Image Text:
H,C,O4(aq) + 2 H,O(l) < C,O4?-(aq) + 2 H,O+(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The odor of fish is due primarily to amines, especially methylamine (CH3NH2). Fish is often served with a wedge of lemon, which contains citric acid. The amine and the acid react forming a product...
-
The following equilibrium constants have been determined for oxalic acid at 25°C: Calculate the equilibrium constant for the following reaction at the same temperature: H2C204(aq)H(aq) HC20 (aq)...
-
The phase diagram for SO2 is shown here. (a) What does this diagram tell you about the enthalpy change in the reaction SO2(I) SO2(g)? (b) Calculate the equilibrium constant for this reaction at 100...
-
Danis Inc is an American firm. The company will receive 626,000 British pounds (GBP) from one of its trading partners in 30 days. The company has obtained an analyst report for possible foreign...
-
What are some of the possible reasons Scott did not seek or receive advice from her immediate supervisor? Sue Ann Scott was a receptionist at the headquarters of a large corporation. A high school...
-
Measurements on ear length were obtained from three populations of corntwo inbred varieties and a randomly pollinated population derived from a cross between the two inbred strains. The phenotypic...
-
With reference to the preceding exercise, find the marginal densities of the two random variables. Data From Preceding Exercise Determining a joint cumulative distribution function Find the joint...
-
Use the information for Indiana Jones Corporation from BE21-9. Assume that for Lost Ark Company, the lessor, Collectibility is reasonably predictable, there are no important uncertainties concerning...
-
Find instances within the last three years where each of the three chosen requirements were violated. Describe each situation, how they violated the requirement, the results of the violation and what...
-
Given the following solutions: (a) 0.1 M NH 3 (b) 0.1 M Na 2 CO 3 (c) 0.1 M NaCl (d) 0.1 M CH 3 CO 2 H (e) 0.1 M NH 4 Cl (f) 0.1 M NaCH 3 CO 2 (g) 0.1 M NH 4 CH 3 CO 2 (i) Which of the solutions are...
-
The base ethylamine (CH 3 CH 2 NH 2 ) has a K b of 4.3 10 4 . A closely related base, ethanolamine (HOCH 2 CH 2 NH 2 ), has a K b of 3.2 10 5 . (a) Which of the two bases is stronger? (b) Calculate...
-
A stone of weight W has specific gravity 2.50? (a) When the stone is suspended from a scale and submerged in water, what is the scale reading in terms of its weight in air? (b) What is the scale...
-
Integrate the following function using onepoint and twopoint numerical integration (Gauss quadrature). The exact integral is equal to 2. Compare the accuracy of the numerical integration with the...
-
Assume your team comprises your organizations Ethics Committee. You receive the following anonymous note: Your team will be determining the best solution to the problem and then communicating it in a...
-
Identify and correct the errors in the following passages. a. Company's are finding it to their advantage to cultivate their suppliers. Partnerships among a company and it's suppliers can yield hefty...
-
The following are emails from various students to Dr. Destiny Sands, who is a professor in the English Department. These students are wondering if Dr. Sands would let them register for her...
-
Use \(1 \times 2\) Gauss rule to evaluate the integral \(I=\iint x y^{2} \mathrm{~d} x \mathrm{~d} y\) over the rectangular region shown in the figure. Explain why the above estimate is or is not...
-
Refer to the information presented in PB2-1 for Coda Industries. Information from PB2-1 (a) Purchased materials on account at a cost of $270,500. (b) Requisitioned materials at a cost of $195,500,...
-
Consider the following cash flows in Table P5.5. (a) Calculate the payback period for each project. (b) Determine whether it is meaningful to calculate a payback period for project D. (c) Assuming...
-
Consider rotation about the C~C bond in ethane. A crude model for torsion about this bond is the free rotor model where rotation is considered unhindered. In this model the energy levels along the...
-
Inspection of the thermodynamic tables in the back of the text reveals that many molecules have quite similar constant volume heat capacities. a. The value of C V, m for Ar (g) at standard...
-
The molar constant volume heat capacity for I 2 (g) is 28.6 J mol 1 K 1 . What is the vibrational contribution to the heat capacity? You can assume that the contribution from the electronic degrees...
-
Question: y = -80 - 26x1 + 47.2x2 y = - 8 0 - 2 6 x 1 + 4 7 . 2 x 2
-
Solve the following inequality. Question: 3.Ix-2I -4 <8 3 . 3 . Ix 2 - 2 I 4 < 8 - 4 < 8
-
Question: 4(x 9)=7(x +5) x 4 ( x 9 ) = 7 ( x + 5 ) x

Study smarter with the SolutionInn App


