Potassium perchlorate is prepared by the following sequence of reactions What mass of Cl 2 (g) is
Question:
Potassium perchlorate is prepared by the following sequence of reactions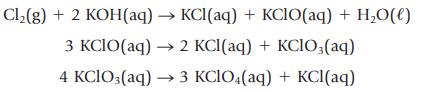
What mass of Cl2(g) is required to produce 234 kg of KClO4?
Transcribed Image Text:
Cl₂(g) + 2 KOH(aq) → KCl(aq) + KCIO(aq) + H₂O(l) 3 KCIO(aq) → 2 KCl(aq) + KCIO3(aq) 4 KClO3(aq) → 3 KClO4(aq) + KCl(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To find the mass of Cl2g required to produce 234 kg of KClO4 we need to work through the stoichiomet...View the full answer

Answered By

Jitendra Tanwar
I did my master from IIT ISN DHANBAD I love to code and solve math problems specially calculas and algebra I will be happy to discuss math problems at master level and interchange math learning by teaching it
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The herbicide trifluralin is prepared by the following sequence of reactions. Identify compound A and deduce the structure of trifluralin. CF3 (CH,CH,CHNH Trifluralin HCompound A CH CIFN204) Cl
-
The herbicide trifluralin is prepared by the following sequence of reactions. Identify compound A and deduce the structure of trifluralin.
-
The most common isotope of uranium, 23892U, produces radon, 22286Rn, through the following sequence of decays: A sample of 23892U will build up equilibrium concentrations of its daughter nuclei down...
-
Examine how effective change communication can reduce resistance in organization ? explain
-
Give three examples showing why a business needs to forecast.
-
A sample of 26 patients with major depressive disorder (MDD) showed an average of five symptoms of the disorder prior to diagnosis. What is the sum of the differences of scores from the mean in this...
-
The combination of a uniform flow and a source can be used to describe flow around a streamlined body called a halfbody. Assume that a certain body has the shape of a half-body with a thickness of...
-
Project Analysis McGilla Golf has decided to sell a new line of golf clubs. The clubs will sell for $700 per set and have a variable cost of $320 per set. The company has spent $150,000 for a...
-
A pitcher throws a 0.60 kg ball of clay at a 4.0 kg block of wood. The clay sticks to the wood on impact, and their joint velocity afterward is 2.5 m/s. What was the original speed of the clay (in...
-
Commercial sodium hydrosulfite is 90.1% Na 2 S 2 O 4 . The sequence of reactions used to prepare the compound is (a) What mass of pure Na 2 S 2 O 4 can be prepared from 125 kg of Zn, 500. g of SO 2 ,...
-
An unknown metal reacts with oxygen to give the metal oxide, MO 2 . Identify the metal if a 0.356-g sample of the metal produces 0.452 g of the metal oxide.
-
K. B. Sayer Furnishings Inc. had the following sales and gross profit percentages for the years 2012-2015. ....................................................Sales................... Gross Profit...
-
Add. Simplify, if possible. 2 7 W +4w+4 + 2 2 W-4
-
This involves one person (or a small team) and can be completed in less than a day. One example is to use torque wrenches instead of adjustable wrenches. This statement BEST describes which of the...
-
After her dentist commented on the damage her practice of vomiting had caused to her teeth, Hilda realized that she had a problem. After seeing her psychiatrist, Hilda was diagnosed with anorexia,...
-
How much force is being experienced by each foot if the bar has a weight of 1682 N and she has a weight of 903 N. You may use 10 m/s2 for gravity, leave the answer to zero decimal places.
-
Describe the manner in which the principles underlying short run input- output and cost- output relationships in production affect the decision making process of enterprises.
-
Where is the current portion of notes payable reported on the balance sheet?
-
The roof of a refrigerated truck compartment is of composite construction, consisting of a layer of foamed urethane insulation (t2 = 50 mm, ki = 0.026 W/m K sandwiched between aluminum alloy panels...
-
Compound A has molecular formula C 7 H 14 O and reacts with sodium borohydride in methanol to form an alcohol. The 1 H NMR spectrum of compound A exhibits only two signals: a doublet (I = 12) and a...
-
Using the information provided below, deduce the structures of compounds A, B, C, and D: 1) EtMgBr 1) O3 (C10H12) (C,H160) 2) H,0 2) DMS (C9H100) [H*), (CH3)2NH AICI3 (-H20)
-
Identify the structures of compounds A to D below, and then identify the reagents that can be used to convert cyclohexene into compound D in just one step. , ,* H,Cro, ] NH,NH, (--0) / D heat
-
You are asked to remove a software and reinstall it. To avoid losing any data, you are asked to complete the works out of business hours. List your major steps. Your assistant added two more hard...
-
1. Briefly explain what GKE is and how it works. 2. What are the main advantages of using a managed service such as GKE over on-premise (non-hosted) solutions? 3. What are the disadvantages of using...
-
Consider a slotted ALOHA system with 20 nodes. Each node transmits a frame in a slot with probability 0.12. A. What is the probability that no node transmits in a slot? Give your answer to 4 decimal...

Study smarter with the SolutionInn App


