Several standard enthalpies of formation (from Appendix L) are given below. Use these data to calculate (a)
Question:
Several standard enthalpies of formation (from Appendix L) are given below. Use these data to calculate
(a) The standard enthalpy of vaporization of bromine.
(b) The energy required for the reaction Br2(g) →2 Br(g). (This is the BrOBr bond dissociation enthalpy.) 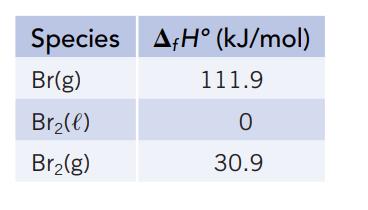
Transcribed Image Text:
Species Br(g) Br₂(l) Br₂(g) AH (kJ/mol) 111.9 0 30.9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
To calculate the standard enthalpy of vaporization of bromine Hvap and the energy required for the r...View the full answer

Answered By

Nyron Beeput
I am an active educator and professional tutor with substantial experience in Biology and General Science. The past two years I have been tutoring online intensively with high school and college students. I have been teaching for four years and this experience has helped me to hone skills such as patience, dedication and flexibility. I work at the pace of my students and ensure that they understand.
My method of using real life examples that my students can relate to has helped them grasp concepts more readily. I also help students learn how to apply their knowledge and they appreciate that very much.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
Use standard enthalpies of formation in Appendix L to calculate enthalpy changes for the following: (a) 1.0 g of white phosphorus burns, forming P 4 O 10 (s) (b) 0.20 mol of NO(g) decomposes to N 2...
-
Use standard enthalpies of formation in Appendix L to calculate enthalpy changes for the following: (a) 0.054 g of sulfur burns, forming SO 2 (g) (b) 0.20 mol of HgO(s) decomposes to Hg() and O 2 (g)...
-
The economic environment of our country currently does not lend itself to being able to successfully run capital campaigns. In a two page document, research and identify 2 successfully capital...
-
A particular LP problem has the following objective function: Maximize profit = $8X1 + $6X2 + $12X3 $2X4 Which variable should enter at the second simplex tableau? If the objective function were...
-
Pique AG shows a balance of 241,140 in the Accounts Receivable account on December 31, 2019. The balance consists of the following. Installment accounts due in...
-
The statement of cash flows for Digital Business Ltd follows. Required (a) Explain the information that would be presented in Note 20 of Digital Business Ltds financial statements. (b) Explain the...
-
Brendan Coleman created and marketed Clinex, a software billing program. Later, Retina Consultants, P. C., a medical practice, hired Coleman as a software engineer. Together, they modified the Clinex...
-
Can you comment to these two posts of my classmates its discussion questions and i need to comment in two of them? To me, what makes someone beautiful changes in stages. My first initial perception...
-
When 0.850 g of Mg was burned in oxygen in a constant-volume calorimeter, 25.4 kJ of energy as heat was evolved. The calorimeter was in an insulated container with 750. g of water at an initial...
-
Isomers are molecules with the same elemental composition but a different atomic arrangement. Three isomers with the formula C 4 H 8 are shown in the models below. The enthalpy of combustion ( c H)...
-
Government officials of Hampstead County ordered a computer near the end of the current fiscal year for $6,400 for the police department. It did not arrive prior to the end of the year. At its final...
-
Although not all clients use A&R for both services, about 7 0 percent do . A&R bills audit services based on billable hours and advising services at a fixed fee. The cost for audit services is...
-
At the point indicated by the star in this figure, should an individual who is already in such a group leave that group to go out on its own? Individual net benefit (benefit - costs) 1 2 3 4 5 Group...
-
1. Select the scientific theory used in several nursing theories to explain the following scenario: One nurse, whose adult son died in the ER 11 years ago, contends that she can never walk into a...
-
Part I: Based on the discussion regarding the pros and cons of free trade policies, please give an informed opinion: What are your thoughts about it? In which circumstances (if any) would you support...
-
In the Plan Solicitation phase, which buyer job task is the most important? Why? Substantiate your answer and be prepared to defend your answer.
-
Golf Carts Inc. must set up an assembly line for golf carts. Forecasts show that 10 units per day should be produced. The plant operates one eight-hour shift each day and runs the line continuously...
-
Match each of the key terms with the definition that best fits it. _______________ A record of the sequence of data entries and the date of those entries. Here are the key terms from the chapter. The...
-
Tri-tert-butylamine cannot be prepared via a reductive amination. Explain.
-
Propose an efficient synthesis for the following transformation: N.
-
Using ammonia as your source of nitrogen, show the reagents you would use to prepare each of the following amines: (a) (b) (c) (d) (e) (f) Z
-
Write the modified commands (with details) to convert shape (A) to shape (B). (7 Marks) 50 (A) R10 Start point- 70,70 88 1-24 88 R10 (B) 50
-
In 2005, Lance Armstrong won the 92nd Tour de France by riding 3608 km in 86 hours, 15 minute s and 2 seconds. Ivan Bosso came in second, 4 minutes and 41 seconds behind Armstrong. Michael Rasmussen...
-
A proton initially has v = 5.01-1.0 +7.0k and then 4.0 s later has v = -3.01-1.0 +8.0k (in meters per second). (a) For that 4.0 s, what is the proton's average acceleration avg in unit-vector...

Study smarter with the SolutionInn App


