The boron trihalides (except BF 3 ) hydrolyze completely to boric acid and the acid HX. (a)
Question:
The boron trihalides (except BF3) hydrolyze completely to boric acid and the acid HX.
(a) Write a balanced equation for the reaction of BCl3 with water.
(b) Calculate ΔrH° for the hydrolysis of BCl3 using data in Appendix L and the following information:
ΔfH° [BCl3(g)] = −403 kJ/mol; ΔfH° [B(OH)3(s)]= −1094 kJ/mol.
Data given in Appendix L
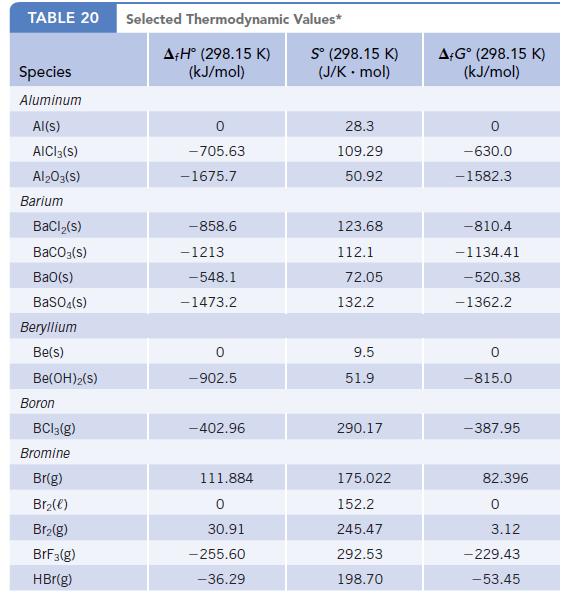
Transcribed Image Text:
TABLE 20 Species Aluminum Al(s) AICI 3(S) Al2O3(S) Barium BaCl₂(s) BaCO3(s) BaO(s) BaSO4(s) Beryllium Be(s) Be(OH)2(S) Boron BC13(g) Bromine Br(g) Br₂(e) Br₂(g) BrF3(g) HBr(g) Selected Thermodynamic A+Hº (298.15 K) (kJ/mol) 0 -705.63 -1675.7 -858.6 -1213 -548.1 -1473.2 -902.5 -402.96 111.884 0 30.91 -255.60 -36.29 Values* Sº (298.15 K) (J/K . mol) 28.3 109.29 50.92 123.68 112.1 72.05 132.2 9.5 51.9 290.17 175.022 152.2 245.47 292.53 198.70 A+Gᵒ (298.15 K) (kJ/mol) -630.0 -1582.3 -810.4 -1134.41 -520.38 -1362.2 0 -815.0 -387.95 82.396 0 3.12 - 229.43 -53.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a Balanced chemical reaction for BCI and water B...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Boron nitride (BN) exists in two forms. The first is a slippery solid formed from the reaction of BCl3 with NH3, followed by heating in an ammonia atmosphere at 750oC. Subjecting the first form of BN...
-
Write a balanced equation for each of the following reactions or reaction sequences. (a) The reaction catalyzed by PFK-2 (b) The conversion of 2 moles of oxaloacetate to glucose (c) The conversion of...
-
Write a balanced equation for the complete oxidation of each of the following, and calculate the respiratory quotient for each substance. (a) Ethanol (b) Acetic acid (c) Stearic acid (d) Oleic acid...
-
Craig's Bowling Inc. operates several bowling centres (for games and equipment sales). The following transactions occurred in November 2017. For each of the following transactions, complete the...
-
Assume that two kinds of buyers purchase contracts from a monopolist who promises to deliver goods in the future. One kind of buyer values the good more highly than the other. The monopolist would...
-
OpenSeas, Inc. is evaluating the purchase of a new cruise ship. The ship would cost $497 million, and would operate for 20 years. OpenSeas expects annual cash flows from operating the ship to be...
-
Make decisions in the situations described in the Ethical Behavior Worksheet. You will not have all the background information on each situation; instead, you should make whatever assumptions you...
-
Grace Herron has just approached a venture capitalist for financing for her new business venture, the development of a local ski hill. On July 1, 2013, Grace was loaned $150,000 at an annual interest...
-
Thompson's Hardware spent $46,370 this year on business insurance alone. If total sales were $765,500, what percent of total sales was spent on business insurance? Round to the nearest tenth.
-
Draw a possible structure for the cyclic anion in the salt K 3 B 3 O 6 and the anion in Ca 2 B 2 O 5 .
-
The element below aluminum in Group 3A is gallium, and there are numerous similarities in the chemistry of these two elements. For example, the hydroxides of both elements are amphoteric. A...
-
It is claimed in this chapter that either price floors or price ceilings reduce the actual quantity exchanged in a market. Use a diagram or diagrams to test this conclusion, and explain the common...
-
In September 2020, Mimi received an offer from a potential buyer to acquire the property and received a deposit of RM17,000. The potential buyer decided not to pursue the acquisition as Covid case...
-
Suppose that Fox Entertainment Group has just made an offer to acquire CKX, the firm that owns American Idol.Prior to the offer, CKX had 30 million shares outstanding that traded at a price of $25,...
-
Develop a policy that outlines what police offi- cers should do if they arrive on a domestic vio- lence call involving a police officer. Outline the investigative and administrative follow-up that...
-
Cheron had the following medical expenses in 2023. Her AGI is $94,000. How much of her medical expenses are deductible, if she itemizes? Medical insurance premiums paid pre tax through her employer's...
-
Suppose that the expected return of the market portfolio is 10% and the risk-free rate is 2%. Suppose a certain stock has a 1 of 1/2 (one half). According to the CAPM, what should the expected return...
-
The newspaper reported last week that Bennington Enterprises earned $34 million this year. The report also stated that the firms return on equity is 16 percent. Bennington retains 80 percent of its...
-
Read the Forecasting Supply Chain Demand Starbucks Corporation case in your text Operations and Supply Chain Management on pages 484-485, then address the four questions associated with the...
-
Figure 2.24 (page 39) shows one of Galileos experiments in which a ball rolls up an incline. A ball that is initially rolling up the incline will roll up to some maximum height and then roll back...
-
A rabbit runs in a straight line with a velocity of +1.5 m/s for a period of time, rests for 10 s, and then runs again along the same line at +0.60 m/s for an unknown amount of time. The rabbit...
-
Figure P2.36 shows the acceleration as a function of time for an object. (a) If the object starts from rest at t - 0, what is the velocity of the object as a function of time? (b) If the object...
-
Plot the sine function (mesh plot and contour plot) whose equation is given by: sinx+y [6] 02313055 and-5 Plot the sine function (mesh plot and contour plot) whose equation is given by: sinx+y over...
-
create a closed card sort game 4:39 AM Sat Oct 7 OPTIMAL OW WORKSHOP Dive deep into card sorting with OptimalSort Introduction Setup > Analysis > optimalworkshop.com Product Solutions Pricing...
-
Explain the top 5 reasons why SCM analytics might fail in your organization? Make your assumptions to answer this question.

Study smarter with the SolutionInn App


