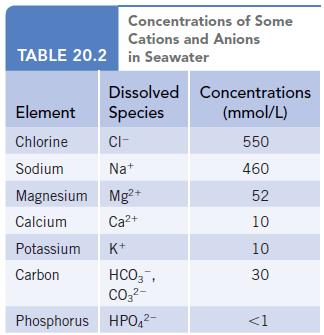
The carbon content in seawater is 30 mmol/L (Table 20.2). This includes both HCO 3 and
Question:
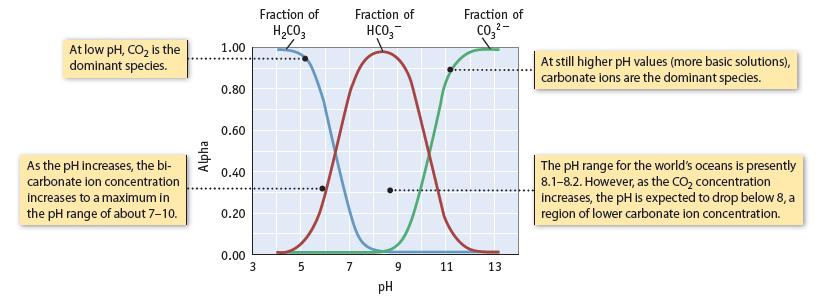
The carbon content in seawater is 30 mmol/L (Table 20.2). This includes both HCO3− and CO32− ions. Knowing the pH of seawater is 8.1, what is the ratio of concentrations of these two ions in seawater? (Figure 20.25.)
Data given in Table 20.2
Data given in Figure 20.25
Transcribed Image Text:
Concentrations of Some Cations and Anions TABLE 20.2 in Seawater Dissolved Concentrations Species (mmol/L) 550 460 52 10 10 Element Chlorine CI- Sodium Na+ Magnesium Mg²+ Calcium Ca²+ Potassium K+ Carbon HCO3, CO3²- Phosphorus HPO4²- 30 <1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
According to HendersonHasselbalch equation PH Pk...View the full answer

Answered By

Evans Cherono
I am an Information Technology Graduate and willing to work on any computer science or IT work to ensure I do my best all the time.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
On the night of July 11, 2007, Akshay Mittal, managing director of Chandpur Enterprises Limited (CEL), was staring at a sheet of numbers to decide on the raw materials requirement for the August...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
According to Electronic Designs 2012 Engineering Salary Survey, the mean base salary of a software engineering manager is $126,417the highest mean among all types of engineers. In contrast, a...
-
In most cities, a Multiple Listing Service (MLS) is used by real estate agents to share information about properties on the market via a computerized database. Agents subscribe to the MLS to list the...
-
You have just completed a grueling 10-day business trip calling on two dozen accounts up and down the West Coast. There were even business meetings combined with social events late into the night and...
-
A new induction motor is put into operation. The initial vibration is acceptable, but after 1 hour of loaded operation, the 1 vibration has increased to an unacceptable level. The motor is...
-
Timken Company specializes in the repair of music equipment and is owned and operated by Secilia Timken. On April 30, 2012, the end of the current year, the accountant for Timken Company prepared the...
-
Describe the situation from either your professional experience or your research in which unethical or fraudulent behavior occurred. If you are describing an example from experience, please do not...
-
Various reagents are added to water supplies to kill pathogens and make the water safe to drink. Among the substances listed below, which does not serve that function? (a) Cl 2 (b) O 3 (c) Ca(ClO) 2...
-
Suppose you find the CO concentration in your home is 10. ppm by volume at 1.00 atm pressure and 25C. What is the concentration in mg/L and in ppm by mass. (The average molar mass for dry air is...
-
Liquid sodium leaves a nuclear reactor at 800C and is used as the energy souce in a steam power plant. The condenser cooling water comes from a cooling tower at 15C. Determine the maximum thermal...
-
The signed-rank statistic can be represented as S + = 1 U 1 + 2 U 2 +.....+ n U n where U i = 1 if the sign of the (x i 0 ) with the ith largest absolute magnitude is positive (in which case i...
-
Show that the ratio of the Laspeyres price index to the Laspeyres quantity index is equal to the ratio of the Paasche price index to the Paasche quantity index.
-
Download the Statement on Standards in Personal Financial Planning Services (SSPFPS) No.1. Review the steps in the PFP process....
-
Jamie OConnor is a second-grade teacher in a rural area. She notices that one of her students, Bobby, consistently comes to school with bruises and cuts. When Jamie asked Bobby about the injuries, he...
-
There are a few situations in which accomplice liability is a legal impossibility. One who abandons his or her intent to participate in the crime may not be considered an accomplice. To succeed,...
-
Suppose a company was going to pay out one-half of its total assets as a cash dividend. What would you expect to happen to the value of the companys stock as a result of the dividend?
-
You are the newly appointed tax practitioner to complete Emilys tax return and have downloaded the prefill report for Emilys tax return (hint, you can read what a prefill report is here (Links to an...
-
The average magnitude of the Poynting vector for sunlight arriving at the top of Earths atmosphere (1.5 10 11 m from the Sun) is about 1.4 kW/m 2 . (a) Compute the average radiation pressure exerted...
-
A surface is placed perpendicular to a beam of light of constant irradiance (I). Suppose that the fraction of the irradiance absorbed by the surface is . Show that the pressure on the surface is...
-
A light beam with an irradiance of 2.00 106 W/m 2 impinges normally on a surface that reflects 70.0% and absorbs 30.0%. Compute the resulting radiation pressure on the surface.
-
You have been asked to estimate the cost of capital for the UTX corporation. The company has 7 million shares and 150,000 bonds outstanding at par value $10,000. In addition, it has $300 million in...
-
MM Corp. has 50,000 shares outstanding with share price of $18. It has debt with market value of $300,000. The equity beta is 1.2 and debt beta is 0.1. The risk-free rate is 2% and the market risk...
-
The market is expected to return 15 percent next year and the risk-free rate is 7 percent. What is the expected rate of return on a stock with a beta of 1.3? The covariance of the market's returns...

Study smarter with the SolutionInn App


