The compound oxygen difluoride is quite reactive, giving oxygen and HF when treated with water: Using bond
Question:
The compound oxygen difluoride is quite reactive, giving oxygen and HF when treated with water: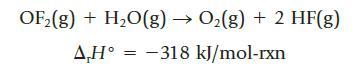
Using bond dissociation enthalpies, calculate the bond dissociation enthalpy of the O—F bond in OF2.
Transcribed Image Text:
OF2(g) + H2O(g) → O2(g) + 2 HF(g) ΔΗ° = −318 kJ/mol-rxn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (5 reviews)
To calculate the bond dissociation enthalpy also known as bond energy of the OF bond in OF2 using bo...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The "plastic" explosive C-4, often used in action movies, contains the molecule cyclotrimethylenetrinitramine, which is often called RDX (for Royal Demolition eXplosive):...
-
QUESTION 20 An equity analyst wants to verify whether there is a variation in the mean rate of return for 8 types of equity. The analyst selected a sample of 142 and conducted an ANOVA test that is...
-
Q. 3. a) Given that Bakkar is just new in the market, what would you describe as his best distribution channel? And what marketing options does he have to advertise and popularize his product while...
-
Two capillary tubes of the same radius r but of lengths & and 1 are fitted horizontally to the bottom of a tall vessel containing a liquid at constant Pressure head and flowing through these tubes....
-
Define the term subsystem. Give an example of a business subsystem, and identify some subsystems with which it relates.
-
The following transactions took place at Fine Fashions Outlet during July 2019. Fine Fashions Outlet uses a perpetual inventory system. Record the transactions in a general journal. Use 8 as the page...
-
Data extracted from a year-end balance sheet are shown below. Compute the working capital for this firm. What would the current ratio be, and what is the significance for this firm? Accounts payable...
-
Do you believe that the Department of Transportations current rules for helping DBEs get highway construction contracts pass the strict scrutiny requirement?
-
What role do epigenetic mechanisms play in modulating stress susceptibility and resilience, and how can this knowledge inform the design of precision medicine approaches for stress management...
-
In boron compounds, the B atom often is not surrounded by four valence electron pairs. Illustrate this with BCl 3 . Show how the molecule can achieve an octet configuration by forming a coordinate...
-
Oxygen atoms can combine with ozone to form oxygen: Using r H and the bond dissociation enthalpy data in Table 8.8, estimate the bond dissociation enthalpy for the oxygenoxygen bond in ozone, O 3 ....
-
We consider a process described by difference equation where the u(t) R is the input, y(t) R the output, and the coefficient vector a(t) R 3 is time-varying. We seek to compute bounds on the...
-
Discuss and explain FIVE (5) trade restrictions that a company must be prepared to face if it chooses to go into international marketing.
-
Discuss five example of global supply chain strategy adopted by Nike in order to support the green economy.
-
(a) Differentiate between the within and between estimators in panel data analysis. (5) (b) Explain the implications of not considering the correlation structure in panel data and how such a...
-
1. A. Discuss why organizations need to experience change?? Explain using scientific proofs. B. Evaluate the following situations in terms of change process and change types. Explain using scientific...
-
1.Please write Marketing Plan for small business of selling chickens. in terms of: Products (chickens fresh and frozen) , Price Promotions/Advertising Place 2. Write the target market and product...
-
Who applies to MBA programs? To help determine the background of the applicants, a sample of 230 applicants to a universitys business school were asked to report their undergraduate degree. The...
-
Describe the Operations (+,,*,/) that can cause negligible addition (NA), error magnification (EM), or subtractive cancellation (SC) in calculating ?((x^2)+1) - x . Give the range of where they might...
-
Why must the amplitudes of the first derivatives of the energy eigenfunctions in the finite depth box and in the adjoining barrier regions have the same value at the boundary?
-
Why must the amplitudes of the energy eigenfunctions in the finite depth box and in the adjoining barrier regions have the same value at the boundary?
-
Explain how a quantum dot can absorb light over a range of wavelengths and emit light over a much smaller range of wavelengths.
-
Give an example of a time where you had to manage your self-presentation or impression in order to be perceived a specific way.What things did you change?
-
Explain the concept of chemical potential in the context of thermodynamics, particularly in relation to the Gibbs-Duhem equation and its significance in predicting phase equilibria in complex...
-
What are the political and economic implications of the trend towards greater media power among so few transnational conglomerates?

Study smarter with the SolutionInn App


