The equation for the oxidation of phosphorus in air is Identify the reactants and products and the
Question:
The equation for the oxidation of phosphorus in air is ![]() Identify the reactants and products and the stoichiometric coefficients. To what do the designations s and g refer?
Identify the reactants and products and the stoichiometric coefficients. To what do the designations s and g refer?
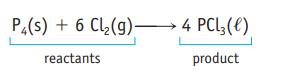
Transcribed Image Text:
P4(s) + 5 O₂(g) → P4O10(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 20% (5 reviews)
In the chemical equation for the oxidation of phosphorus in air P4s 5 O2g P4O10s The reactants ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Consider a process where some super heroes and a enemy are fighting to recover planets that the enemy has controlled, for which the enemy or the super heroes can win battles, this information is...
-
On the 16th of April, the company paid 6,000 for one year insurance starting from the 1st of April. Prepare Journal Entry?
-
The oxidation of glucose provides the principal source of energy for animal cells. Assume the reactants are glucose [C6H12O6(s)] and oxygen [O2(g)]. The products are CO2(g) and H2O(l). (a) Write a...
-
5 Question 42 (2.5 points) Consider a put option that gives the long position the right to sell the underlying asset for $12.34 in 5.67 years. The continuously compounded risk free rate of interest...
-
The nation of Acirema is small and unable to affect world prices. It imports peanut at the price of $10 per bag. The demand curve is D = 400 10P The supply curve is S = 50 + 5P. Determine the free...
-
The following account balances are taken from the records of the Faraway Travel Agency: Faraway extends credit terms requiring full payment in 60 days, with no discount for early payment. Required 1....
-
A double acting reciprocating pump delivering 80 litres per second has the following specifications : Diameter of piston = 300 mm; Stroke = 600 mm; Speed = 80 rpm; Suction head = 5 m; Delivery head =...
-
Topez Company was started on January 1, 2014. During 2014, the company experienced the following three accounting events: (1) earned cash revenues of $14,500, (2) paid cash expenses of $9,200, and...
-
The Janobi Company has three product lines of beer mugs-A, B, and C-with contribution margins of $5, $4, and $2, respectively. The president foresees sales of 231,000 units in the coming period,...
-
Strontium has four stable isotopes. Strontium-84 has a very low natural abundance, but 86 Sr, 87 Sr, and 88 Sr are all reasonably abundant. Knowing that the atomic weight of strontium is 87.62, which...
-
Carry out the following operations. Provide the answer with the correct number of significant figures. (a). (1.52)(6.21 10 3 ) (b). (6.217 103)(5.23 10 2 ) (c). (6.217 103) (5.23 10 2 ) (d)...
-
Let Z 2 be an algebraic closure of Z 2 , and let , Z 2 be zeros of x 3 + x 2 + 1 and of x 3 + x 2 + 1, respectively. Using the results of this section, show that Z 2 () = Z 2 ().
-
Consider the following information: Raw materials inventory, beginning $4,000 Raw materials inventory, ending 3,000. Net Purchases 25,000 Freight out 500% Decrease in Work in Process inventory Wip...
-
What would happen if we breach the conditions of the social licence to operate in Australia. Does this mean to compliance risk? What could be happened when there is a revocation of the organisation's...
-
The income statement is an important financial statement because it provides investors, creditors, and managers with information that helps them predict the amount, timing, and uncertainty of future...
-
Blue Industries purchased a machine from Calico Corporation on October 1, 2016. In payment for the $144,000 purchase, Blue issued a one-year installment note to be paid in equal monthly payments at...
-
Complete the following table: Note: Do not round intermediate calculations. Round your final answers to the nearest cent. Item List price Sony digital camera 318 Chain discount 8/4/2 Trade Net price...
-
Assume the same information as for Problem SA-1. Instead of using the equity method, Schinzer uses the fair value option to record the investment in Fowler. The fair value of the investment in Fowler...
-
If someone's Z-score for a variable was 0.67. Their score is a significant extreme score. Their score is not significant. O Their score is slightly above average. O Their score is an outlier.
-
In the experiment shown in Figure 2.4a and 2.4b, ÎU surroundings < 0, but ÎT surroundings > 0. Explain how this is possible. Figure 2.4 Electrical generator Mass Heating- coil Bunsen...
-
What is wrong with the following statement? Burns caused by steam at 100C can be more severe than those caused by water at 100C because steam contains more heat than water. Rewrite the sentence to...
-
Calculate the number of molecules per m 3 in an ideal gas at the standard temperature and pressure conditions of 0.00C and 1.00 atm.
-
Pavlos, Inc., is expecting a period of intense growth, so it has decided to reduce its annual dividend by 10 percent a year for the next three years. After that, it will maintain a constant dividend...
-
Requirement Determine the amount and type of financing component in the following contracts. (Click the icon to view the independent contracts.) Future Value of $1 table Future Value of an Ordinary...
-
Studying other cultures through a humanistic lens allows people to understand how different cultures came about and how and why people behave differently from one place to another (Lombrozo, 2015)....

Study smarter with the SolutionInn App


