The equation for the reaction of phosphorus and chlorine is If you use 8000 molecules of P
Question:
The equation for the reaction of phosphorus and chlorine is ![]() If you use 8000 molecules of P4 in this reaction how many molecules of Cl2 are required to consume the P4 completely?
If you use 8000 molecules of P4 in this reaction how many molecules of Cl2 are required to consume the P4 completely?
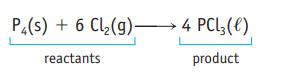
Transcribed Image Text:
P4(s) + 6 Cl₂(g) → 4 PCl3(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
The balanced chemical equation provided is P4s 6 Cl2g 4 PCl3l In this equation e...View the full answer

Answered By

Raunak Agarwal
Teaching is my hobby and now my profession. I teach students of CA and CFA(USA) in batches of 100 students and have a 5 year experience.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Use the subset of 401KSUBS.RAW with fsize = 1; this restricts the analysis to single person households; see also Computer Exercise C4.8. (i) What is the youngest age of people in this sample? How...
-
Use the estimator in the myfico website to estimate the FICO credit score for the following person: -Never had a budget. Not sure where his money is going. Regularly uses credit cards to pay for...
-
The problem requires you to use File C03 on the computer problem spreadsheet. Diction Publishing estimates that it needs $500,000 to support its expected growth. The underwriting fees charged by the...
-
Two slot machines offer to double your money 3 times out of 5. Machine A takes $10 bets and Machine B takes $100 bets on each occasion. A risk-averse investor prefers to bet on A) Machine A B)...
-
For a small country like the Philippines, a move to free trade would have huge advantages. It would let consumers and producers make their choices based on the real costs of goods, not artificial...
-
What is redundancy and how can it improve product design?
-
Show that the log-likelihood in equation (12.2) has a maximum at \(\widehat{\mu}=\bar{y}\). n L() Inf(y,) = (-+y; In - In y;!). i=1 i=1 (12.2)
-
The following is information for three local auto dealers: a. Using the information given in the above table, construct income statements for each company and the industry average. Assume that each...
-
There are 6 ethical values that marketers are expected to uphold, and these are: Honesty - Be forthright in dealings and offer value and integrity. Responsibility - Accept the consequences of...
-
Which of the following compounds has the highest mass percent of chlorine? (a) BCl 3 (b) AsCl 3 (c) GaCl 3 (d) AlCl 3 (e) PCl 3
-
Write an equation from the following description: reactants are gaseous NH 3 and O 2 , products are gaseous NO 2 and liquid H 2 O, and the stoichiometric coefficients are 4, 7, 4, and 6,...
-
Name and describe the four types of inventory most organizations maintain.
-
In the past seven years, Sarah's uncle has been paying her monthly allowance of $1,000 in arrear, directly deposited into Sarah's bank account, with an interest rate of 5% p.a. compounded monthly....
-
1. Compensation income earners may deduct the following from their gross income to arrive at taxable income: a. Itemized Deductions b. Optional Standard Deductions c. First P250,000 d. None of the...
-
Tom Douglas is a famous Seattle restaurant entrepreneur. He has been so successful that he now just angel invests into new concepts that approach him. Recently a slew of entrepreneurs have reached...
-
Fire Tint, Inc. (FTI) applies tint to airplane canopies (front windows) manufactured by other companies. When the canopy manufacturer asks FTI to tint a canopy, FTI tracks the manufacturer, model...
-
You have been hired as a consultant by Steady-State Life Insurance Company. Steady-State specializes in the sale of Guaranteed Investment Contracts (GICs) which guarantee clients a specific dollar...
-
Test the following sequence of observations by using the runs test and α = .05 to determine whether the process produced randomresults. MNNNNM NMM NNNNNN NNNNN M
-
Suppose the S&P 500 futures price is 1000, = 30%, r = 5%, = 5%, T = 1, and n = 3. a. What are the prices of European calls and puts for K = $1000? Why do you find the prices to be equal? b. What...
-
For each of the compounds below, locate the lone pair adjacent to a positive charge and draw the resonance structure: a. b. c. N.
-
Assume the following simplified dependence of the pressure in a ventricle of the human heart as a function of the volume of blood pumped. P s , the systolic pressure, is 120. mm Hg, corresponding to...
-
Electrical current is passed through a resistor immersed in a liquid in an adiabatic container. The temperature of the liquid is varied by 1C. The system consists solely of the liquid. Does heat or...
-
1. Ryan is investing $9000 in a CD at a bank. If the bank uses simple interest and the bank pays 3.1% annually, how much will the CD be worth in total at the end of 5 years when the CD matures? Jim...
-
What strategies can organizations implement to recognize and reward employees who consistently demonstrate advanced levels of Organizational Citizenship Behavior (OCB) ?
-
discuss the potential challenges and barriers that may hinder employees from engaging in advanced Organizational Citizenship Behavior (OCB), and how can these obstacles be overcome?

Study smarter with the SolutionInn App


