The following reaction can be used to prepare iodine in the laboratory. (a) Determine the oxidation number
Question:
The following reaction can be used to prepare iodine in the laboratory.
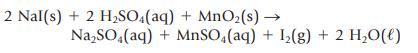
(a) Determine the oxidation number of each atom in the equation.
(b) What is the oxidizing agent, and what has been oxidized? What is the reducing agent, and what has been reduced?
(c) Is the reaction product-favored or reactant-favored?
(d) Name the reactants and products.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





