The formula for nitryl chloride is ClNO 2 (in which N is the central atom). (a) Draw
Question:
The formula for nitryl chloride is ClNO2 (in which N is the central atom).
(a) Draw the Lewis structure for the molecule, including all resonance structures.
(b) What is the N—O bond order?
(c) Describe the electron-pair and molecular geometries, and give values for all bond angles.
(d) What is the most polar bond in the molecule? Is the molecule polar?
(e) The computer program used to calculate electrostatic potential surfaces gave the following charges on atoms in the molecule:
A = −0.03,
B = −0.26, and
C = +0.56.
Identify the atoms A, B, and C. Are these calculated charges in accord with your predictions?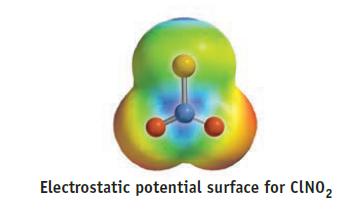
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





