The HaberBosch process for the production of ammonia is one of the key industrial processes in developed
Question:
The Haber–Bosch process for the production of ammonia is one of the key industrial processes in developed countries.
N2(g) + 3 H2(g) ⇄ 2 NH3(g)
(a) Calculate ΔrG° for the reaction at 298 K, 800 K, and 1300 K. Data at 298 K are given in Appendix L. Data for the other temperatures are as follows:

How does the ΔrG°change with temperature?
(b) Calculate the equilibrium constant for the reaction at 298 K, 800 K, and 1300 K.
(c) At what temperature (298 K, 800 K, or 1300 K) is the mole fraction of NH3 the greatest?
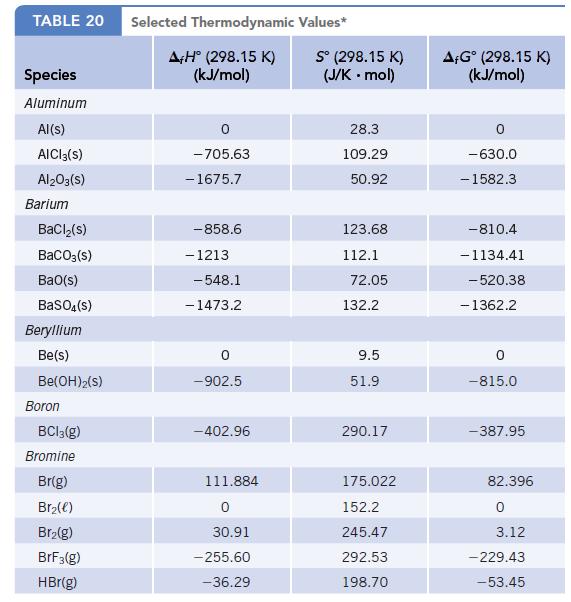
Data given in Appendix L
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





