The reaction of methane and water is one way to prepare hydrogen for use as a fuel:
Question:
The reaction of methane and water is one way to prepare hydrogen for use as a fuel: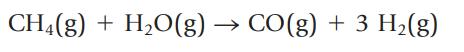
If this reaction has a 37% yield under certain conditions, what mass of CH4 is required to produce 15 g of H2?
Transcribed Image Text:
CH₂(g) + H₂O(g) CO(g) + 3 H₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To find the mass of CH4 required to produce 15 g of H2 with a 37 yield you need to calculate the the...View the full answer

Answered By

Kainat Shabbir
i am an experienced qualified expert with a long record of success helping clients overcome specific difficulties in information technology, business and arts greatly increasing their confidence in these topics. i am providing professional services in following concerns research papers, term papers, dissertation writing, book reports, biography writing, proofreading, editing, article critique, book review, coursework, c++, java, bootstarp, database.
5.00+
184+ Reviews
255+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The reaction of methane and water is one way to prepare hydrogen for use as a fuel: If you begin with 995 g of CH 4 and 2510 g of water, (a) Which reactant is the limiting reactant? (b) What is the...
-
Methane is generated via the anaerobic decomposition (biological degradation in the absence of oxygen) of solid waste in landfills. Collecting the methane for use as a fuel rather than allowing it to...
-
Hydrogen is produced commercially by the reaction of methane with steam: CH4(g) + H2O(g) CO(g) + 3H2(g) a. Calculate Ho and So for this reaction (use the data in Appendix 4). b. What temperatures...
-
Write a Fortran program that creates an integer array with values -123, -4, 5, 67, 890, and 12345. Prints out the array in several formatted ways. a. Print each element, on its own line, using a...
-
Give two examples from the Internet for each of the different revenue models used in e-commerce.
-
What is the role of equations in this book?
-
Figure P5.115 shows a pump testing setup. Water is drawn from a sump and pumped through a pipe containing a valve. The water is discharged into a catch tank sitting on a scale. During a test run,...
-
Applin Corporation has a desired rate of return of 8 percent. Troy Anderson is in charge of one of Applins three investment centers. His center controlled operating assets of $5,000,000 that were...
-
An adult helping her child learn to ride a bike, applies a net force of 4.32 newtons to the child on the bike for 2.40 seconds. How much momentum does the child and his bike gain after being pushed...
-
Methanol, CH 3 OH, can be prepared from carbon monoxide and hydrogen. What mass of hydrogen is required to produce 1.0 L of CH 3 OH (d = 0.791 g/mL) if this reaction has a 74% yield under certain...
-
Black smokers are found in the depths of the oceans. Thinking that the conditions in these smokers might be conducive to the formation of organic compounds, two chemists in Germany found the...
-
Use the definition of continuity to investigate whether g(x) is continuous at x = -2. Show your work. [6 - x if x < -2 if x>-2 g(x)
-
Using only the input data highlighted in green on the tab create a macro in Visual Basic for Applications (VBA) that uses a For Next loop to calculate the Price (at time 0) of a semi-annual coupon...
-
Superior Micro Products uses the weighted-average method of process costing. Data for the Assembly Department for May appear below: Work in process, May 1 Cost added during May Equivalent units of...
-
Edgar and search for the Amazon annual report ( 1 0 - K ) for the year ended December 3 1 , 2 0 1 9 , using EDGAR ( Electronic Data Gathering, Analysis, and Retrieval system ) . Visit www . sec . gov...
-
Isai is married and has two children who each have a spouse. Isai also has two grandchildren, and two close friends. He would like to gift the maximum amount to each child, child's spouse,...
-
Over the past year private equity firms have been extremely active in financing management by outs of under performing or undervalued businesses. What risk factors might pertain to a private equity...
-
In the recording of depreciation expense, which account is credited?
-
The sales department of P. Gillen Manufacturing Company has forecast sales in March to be 20,000 units. Additional information follows: Finished goods inventory, March 1 . . . . . . . . . . . . . . ....
-
Identify the reagents necessary to accomplish each of the following transformations: SO3H Br ZON i NH2
-
Predict the change in the partial pressure of CO2 as O2 is removed from the reaction vessel at constant pressure and temperature. CO(g) + 1/2O 2 (g) CO 2 (g) at equilibrium for which H o R = 283.0...
-
Calculate the maximum expansion work that is available in carrying out the combustion reactions in Example Problems 6.1 and 6.2. Explain both the magnitude and the sign of the work.
-
The root mean square speed of ????2 molecules at 25 degrees Celsius is about 1.6 km/s. What is the root mean square speed of an ????2 molecule at 25 degrees Celsius?
-
The inverse of the collision rate, 1/z, is the average time between collisions of gas particles. Calculate the average time between collisions of an Xe atom in 1.00 mol of Xe at STP.
-
Crane Company sells goods to Ivanhoe Company during 2025. It offers Ivanhoe the following rebates based on total sales to Ivanhoe. If total sales to Ivanhoe are 9,500 units, it will grant a rebate of...

Study smarter with the SolutionInn App


