The solid-state structure of silicon carbide is shown below. (a) How many atoms of each type are
Question:
The solid-state structure of silicon carbide is shown below.
(a) How many atoms of each type are contained within the unit cell? What is the formulas of silicon carbide?
(b) Knowing that the Si—C bond length is 188.8 pm (and the Si—C—Si bond angle is 109.5°), calculate the density of SiC.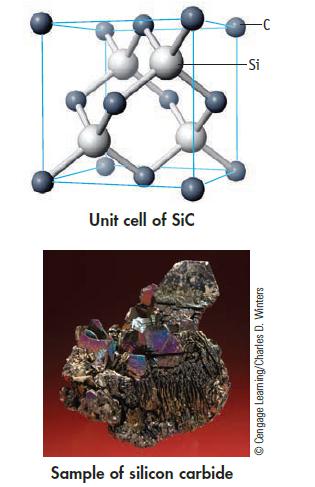
Transcribed Image Text:
Unit cell of Sic Sample of silicon carbide -Si Ⓒ Cengage Learning/Charles D. Winters
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
a In the unit cell of silicon carbide SiC there are four silicon Si atoms and four carbon C atoms Ea...View the full answer

Answered By

Umber Talat
I am providing full time mentoring and tutoring services in Business Finance, Contemporary issue in Global Economy, Quantitative Techniques, Principles of Marketing, strategic marketing, International Marketing, Organizational Behavior (OB), Consumer Behavior, Sales Force Management, Strategic Brand Management, Services Marketing, Integrated Marketing Communication (IMC), Principles of Management, General Management, Strategic Management, Small and Medium Enterprise Management, Innovation Management, Change Management, Knowledge Management, Strategic Planning, Operations Management, Supply Chain Management, Logistics Management, Inventory management, Total Quality Management (TQM), Productions Management, Project Management, Production Planning, Human Resource Management (HRM), Human Resource Development, Strategic HRM, Organizational Planning, Performance and Compensation Management, Recruitment and Selection, Organizational Development, Global Issues in Human Resource Management, Retail Marketing, Entrepreneurship, Entrepreneurial Marketing, International Business, Research Methods in Business, Business Communication, Business Ethics.
4.70+
158+ Reviews
236+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
(a) Figure 22.37 shows eight corner-sharing ReO 6 octahedra in the solid state structure of ReO 3 . From this, derive a diagram to show the unit cell of ReO 3 . Explain the relationship between your...
-
What are the different relays that employed for protection of apparatus and transmission lines?
-
In 1998, Boston Beer produced more than two dozen styles of beer. Then a few years later it was down to just a few. Now its up to more than 21 again. Do you see any problems with this?
-
What are the important items of a budget? Why?
-
What is the purpose of timelines in an investigation?
-
Hazlett, Inc., operates at capacity and makes plastic combs and hairbrushes. Although the combs and brushes are a matching set, they are sold individually and so the sales mix is not 1: 1. Hazletts...
-
2 The Fourier series of the square signal shown below is: 2 f(t) = sin(wo) + [3Pts] 2 2 2 sin(3w0) + sin(5w0) + sin(7wo) + -sin(9wo) T 3 5 7 9 2 + sin(11w0) + ... 11 Plot the first five terms of the...
-
Consider the three types of cubic units cells. (a) Assuming that the spherical atoms or ions in a primitive cubic unit cell just touch along the cubes edges, calculate the percentage of occupied...
-
The solid-state structure of silicon is shown below. (a) Describe this crystal as pc, bcc, or fcc. (b) What type of holes are occupied in the lattice? (c) How many Si atoms are there per unit cell?...
-
Calgary Airlines routinely overbooks its flight from Calgary to Vancouver. Overbooking discounted seats can be expensive because providing a bumped passenger with a last-minute flight on a competing...
-
Rizzo Corporation has 30,000 shares of $50 par value, 4%, preferred stock (P/S) and 150,000 shares of $5 par value common stock (C/S) outstanding at year end,2017. All events are in chronological...
-
Consider the following information: Cost of goods sold: $200,000 Income tax expense: $12,000 Research and development expenses: $42,000 Interest expense: $9,000 Net Sales: $702,000 Selling, general,...
-
Barrymore Industries has monthly fixed costs totaling $30,000 and variable costs of $5 per unit. Each unit of product is sold for $20. What is the break-even point in units? Barrymore Industries has...
-
What is the benefit of knowing generalizations about a target market? What is the benefit of knowing stereotypes that your culture possesses about the culture of the target market?
-
What are 2 ways by that president has authority over executive agencies?
-
At the end of the year, Molly Enterprises estimates the uncollectible accounts expense to be 0.8 percent of net sales of $3,787,500. The current credit balance of Allowance for Uncollectible Accounts...
-
Which task is performed by a book-keeper? A. Analysing the trading results B. Entering transactions in the ledger C. Preparing year-end financial statements D. Providing information for...
-
The 8-mm-diameter bolt is made of an aluminum alloy. It fits through a magnesium sleeve that has an inner diameter of 12 mm and an outer diameter of 20 mm. If the original lengths of the bolt and...
-
An acetal polymer block is fixed to the rigid plates at its top and bottom surfaces. If the top plate displaces 2 mm horizontally when it is subjected to a horizontal force P = 2 kN, determine the...
-
The rigid bar is pinned at A and supported by two aluminum rods, each having a diameter of 1 in., a modulus of elasticity E al = 10(10 3 ) ksi, and yield stress of (Ï Y ) al = 40 ksi. If the bar...
-
ces Direct labor-hours Machine-hours Fixed manufacturing overhead cost Variable manufacturing overhead per machine-hour Variable manufacturing overhead per direct labor-hour 35,000 Department Molding...
-
Define what is meant by an asset and a liability. Give an example of each.
-
Explain why if assets are valuable resources and asset accounts have debit balances why do expense accounts also have debit balances. What is meant by the normal balance?

Study smarter with the SolutionInn App


