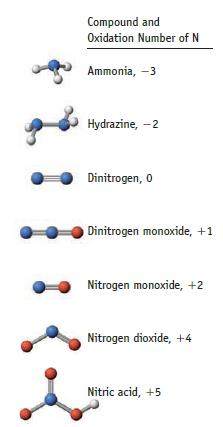
The structure of nitric acid is illustrated on page 990. (a) Why are the NO bonds the
Question:
The structure of nitric acid is illustrated on page 990.
(a) Why are the N—O bonds the same length, and why are both shorter than the N—OH bond length?
(b) Rationalize the bond angles in the molecule.
(c) What is the hybridization of the central N atom? Which orbitals overlap to form the N—O π bond?
Data given on page 990
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





