Tin(IV) oxide, cassiterite, is the main ore of tin. It crystallizes in a rutile-like unit cell with
Question:
Tin(IV) oxide, cassiterite, is the main ore of tin. It crystallizes in a rutile-like unit cell with tin(IV) ions taking the place of Ti4+ ions (Study Question 12.6). (The O2− ions marked x are wholly within the unit cell.)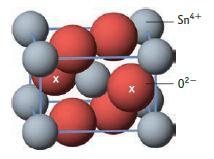
(a) How many tin(IV) ions and oxide ions are there per unit cell of this oxide?
(b) Is it thermodynamically feasible to transform solid SnO2 into liquid SnCl4 by reaction of the oxide with gaseous HCl? What is the equilibrium constant for this reaction at 25°C?
Data given in Question 12.6
Rutile, TiO2, crystallizes in a structure characteristic of many other ionic compounds. How many formula units of TiO2 are in the unit cell illustrated here? (The oxide ions marked by an x are wholly within the cell; the others are in the cell faces.)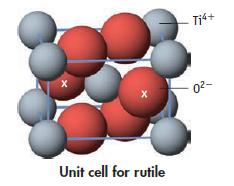
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





