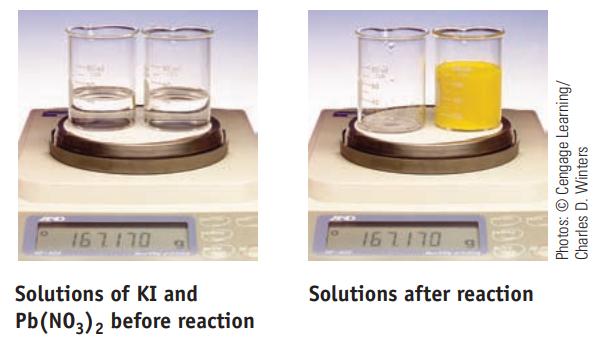
Two beakers sit on a balance; the total mass is 167.170 g. One beaker contains a solution
Question:
Two beakers sit on a balance; the total mass is 167.170 g. One beaker contains a solution of KI; the other contains a solution of Pb(NO3)2. When the solution in one beaker is poured completely into the other, the following reaction occurs:
What is the total mass of the beakers and solutions after reaction? Explain completely
![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





