Use K sp values to decide which compound in each of the following pairs is more soluble.
Question:
Use Ksp values to decide which compound in each of the following pairs is more soluble. (Appendix J.)
(a) AgBr or AgSCN
(b) SrCO3 or SrSO4
(c) AgI or PbI2
(d) MgF2 or CaF2
Data given in Appendix J

Transcribed Image Text:
TABLE 18A Solubility Product Constants at 25 °C Cation Compound Cation Ba²+ *BaCrO Hg₂²+ 2+ BaCO3 BaF₂ *BaSO4 Ca²+ Cut, Cu²+ Au+ Fe²+ Pb²+ Mg²+ Mn²+ CaCO3 (calcite) *CaF2 *Ca(OH)2 CaSO4 CuBr Cul Cu(OH)2 CuSCN AuCl FeCO3 Fe(OH)2 PbBr₂ PbCO3 PbCl₂ PbCrO₁ PbF2 Pbl₂ Pb(OH)2 PbSO4 MgCO3 MgF2 Mg(OH)2 MnCO3 *Mn(OH)2 Ksp 1.2 x 10-10 2.6 × 10-9 1.8 x 10-7 1.1 x 10-10 3.4 x 10-9 5.3 x 10-11 5.5 x 10-5 4.9 x 10-5 6.3 x 10-9 1.3 x 10-12 2.2 x 10-20 1.8 x 10-13 2.0 x 10-13 3.1 x 10-11 4.9 × 10-17 6.6 x 10-6 7.4 x 10-14 1.7 x 10-5 2.8 x 10-13 -13 3.3 x 10-8 9.8 x 10-9 1.4 x 10-15 2.5 x 10-8 6.8 x 10-6 5.2 x 10-11 5.6 x 10-12 2.3 × 10-11 1.9 x 10-13 Ni²+ Ag+ Sr²+ TI+ Zn²+ Compound *Hg₂Br₂ Hg₂Cl₂ *Hg2l2 Hg2SO4 NICO3 Ni(OH)2 *AgBr *AgBrO3 AgCH3CO₂ AgCN Ag₂CO3 *Ag₂C₂04 *AgCl Ag₂ CrO4 *Agl AgSCN *Ag₂SO4 SrCO3 SrF₂ SrSO4 TIBr TICI TII Zn(OH)₂ Zn(CN)₂ Ksp 6.4 x 10-23 1.4 x 10-18 2.9 x 10-29 6.5 x 10-7 1.4 x 10-7 5.5 x 10-16 5.4 x 10-13 5.4 x 10-5 1.9 x 10-3 6.0 x 10-17 8.5 x 10-12 5.4 x 10-12 1.8 x 10-10 1.1 x 10-12 8.5 x 10-17 1.0 x 10-12 1.2 x 10-5 5.6 x 10-10 4.3 × 10-9 3.4 x 10-7 3.7 x 10-6 1.9 x 10-4 5.5 x 10-8 3 x 10-17 8.0 x 10-12
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
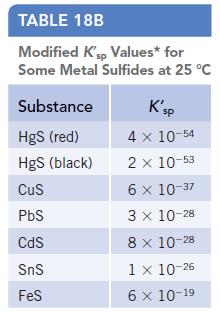
Use K sp values to decide which compound in each of the following pairs is more soluble. (Appendix J.) (a) PbCl 2 or PbBr 2 (b) HgS or FeS (c) Fe(OH) 2 or Zn(OH) 2 Data given in Appendix J TABLE 18A...
-
Which compound in each of the following pairs of compounds is the more soluble one? a. Silver chloride or silver iodide b. Magnesium hydroxide or copper(II) hydroxide
-
Which compound in each of the following pairs would have the higher boiling point? Explain your answers. (a) (b) (c) (d) (e) (f) (g) (h) Hexane, CH3(CH2)4CH3, or nonane, CH3(CH2)7CH3 (i) OH or HO OH...
-
Table P-23 contains Southwest Airlines' quarterly income before extraordinary items ($MM) for the years 1988-1999. a. Plot the income data as a time series and describe any patterns that exist. b. Is...
-
1. Given the facts of this case, should John have been discharged? Explain. 2. Should the sales representatives of AEM be held to a higher standard of personal conduct than sales representatives for...
-
The following transactions for Kramer Corporation occurred during December of the current year. Follow the step-by-step instructions to enter the transactions, process the adjusting entries, and...
-
On a summer day the air temperature is \(30^{\circ} \mathrm{C}\) and the relative humidity is \(55 \%\). Water evaporates from the surface of a lake at a rate of \(0.08 \mathrm{~kg} / \mathrm{h}\)...
-
Circuit Systems, Inc., located in northern California, is a company that produces integrated circuit boards for the microcomputer industry. In addition to salaried management and office staff...
-
1.If variable manufacturing overhead is applied to production on the basis of direct labor-hours and the direct labor efficiency variance is unfavorable, will the variable overhead efficiency...
-
Calculate the molar solubility of silver thiocyanate, AgSCN, in pure water and in water containing 0.010 M NaSCN.
-
If 55 mg of lead(II) sulfate is placed in 250 mL of pure water, does all of it dissolve? If not, how much dissolves?
-
Let f (x) and g(x) be continuous functions on [0, 1], Prove: (a) (b) 1/2 71/2
-
Compute the taxable income for 2019 under each of the following circumstances: a. Jim is married and files a joint return. Jim and his wife have two dependent children. They have adjusted gross...
-
During the last few years, many companies have suffered major trading losses because of the poor economic climate. Assume your firm has found itself in this situation and is considering a major...
-
Quantitative Risk Assessment. Assume the following information: Calculate the overall risk factor for this project. Would you assess this level of risk as low, moderate, or high? Why? Probability of...
-
The file ...birth-death.R contains the data compiled by Phillips and Feldman (1973) on the month of birth and the month of death of 348 'famous Americans'. Investigate whether the month of death is...
-
Syco SA, a distributor of food and food-related products, has announced it has signed an interest rate swap. The interest rate swap effectively converts the companys 100 million, 4.6 per cent...
-
Refer to the information presented in M4-6. Suppose that Acoma manufacturers only the two products mentioned and they consume 100 percent of the companys quality inspections. Using activity...
-
Refrigerant R-12 at 30C, 0.75 MPa enters a steady flow device and exits at 30C, 100 kPa. Assume the process is isothermal and reversible. Find the change in availability of the refrigerant.
-
Explain why two magnetic fields, a static field and a radiofrequency field, are needed to carry out NMR experiments. Why must the two field directions be perpendicular?
-
Explain the difference in the mechanism that gives rise to through-space dipoledipole coupling and through-bond coupling.
-
Predict the number of chemically shifted + 1H peaks and the multiplet splitting of each peak that you would observe for diethyl ether. Justify your answer.
-
Add. 6 2-3x + x-l Simplify your answer as much as possible.
-
During a research experiment, it was found that the number of bacteria in a culture grew at a rate proportional to its size. At 1 0 : 0 0 AM there were 6 , 0 0 0 bacteria present in the culture. At...
-
4) Prove, algebraically, that x +9 =1+- x +8 1 x +8 where x-2 .

Study smarter with the SolutionInn App


