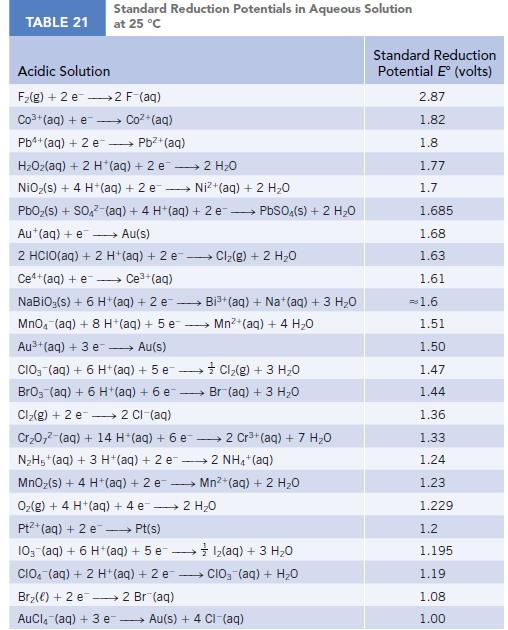
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following
Question:
Use the table of standard reduction potentials (Appendix M) to calculate ΔrG° for the following reactions at 298 K.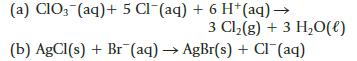
Data given in Appendix M
Transcribed Image Text:
(a) CIO3(aq) + 5 Cl(aq) + 6 H+ (aq) → 3 Cl₂(g) + 3 H₂O(l) (b) AgCl(s) + Br (aq) → AgBr(s) + Cl(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a The relation between Gibbs free energy and ...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following reactions at 298 K. Data given in Appendix M (a) 3 Cu(s) + 2NO3(aq) + 8 H+ (aq) 3 Cu+ (aq) + 2 NO(g) +...
-
Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K: (a) Fe(s) + Ni2+(aq) Fe2+ (aq) + Ni(s) (b) Co(s) + 2 H+...
-
Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K: (a) Cu(s) + 2 Ag+ (aq) Cu2+ (aq) + 2 Ag(s) (b) 3 Ce4+...
-
Built-Tite uses job order costing. The T-account below summarizes Factory overhead activity for the current year. Factory Overhead Debit Credit 16,200 106,600 25,200 60,200 1. Compute total applied...
-
Southern Rail Inc. is considering acquiring equipment at a cost of $442,500. The equipment has an estimated life of 10 years and no residual value. It is expected to provide yearly net cash flows of...
-
The shareholders equity of Henry Byce Ltd. at the end of 2020 and 2019 appeared as follows: Byce began operations in 2018. The 50,000 preferred shares issued have been outstanding since 2018. During...
-
1. Two cards are selected, without replacing the first card, from a standard deck of 52 playing cards. Find the probability of selecting a king and then selecting a queen. 2. A coin is tossed and a...
-
Scott Bestor is an accountant for Westfield Company. Early this year, Scott made a highly favorable projection of sales and profits over the next 3 years for Westfields hot-selling computer PLEX. As...
-
4. How are organisation health and safety procedures, health and safety signage and safe work practices going to be monitored at ANIBT Hotel? Include how you will monitor ongoing compliance. 5. What...
-
In 2005, global SO 2 emission was estimated to be 12.83 Gg (gigagrams). According to the EPA, 71% of SO 2 emissions into the atmosphere is from coalfired power plants. How much coal (in metric tons)...
-
Calculate equilibrium constants for the following reactions at 298 K. Indicate whether the equilibrium as written is reactant- or product-favored at equilibrium. (a) 2 Cl(aq) + Br(e) (b) Fe+ (aq) +...
-
In Exercises 4547, solve each formula for the specified variable. vt + gt = s for g
-
1) Count to 15 in decimal, hexadecimal and binary, in a column to the right 2) What is the decimal equivalent for the hexadecimal number 1BC 3) What is the decimal equivalent for the binary number...
-
How does the painter's manipulation of chiaroscuro and perspective contribute to the evocative imagery that imbues their work with layers of symbolic meaning and psychological depth?
-
PLANNING BUSINESS MESSAGES. Topic 1: Following the 3-x-3 writing process Topic 2: Analyzing the Purpose and anticipating the audience Topic 3: Improving the Tone and Clarity of a Message. Describe...
-
How does the poet's adept use of imagery serve to transmute the ineffable essence of human experience into a vivid tapestry of sensory perception and emotional resonance?
-
What actions could JP Chase & Morgan have used to lessen the impact of the 2 0 1 3 financial loss? Explain
-
At a camp cafeteria teenagers were randomly given a tall skinny glass or a short wide glass. As they proceeded through the line they loaded up on the food they wanted and poured whatever drink they...
-
1. Which of the four major types of information systems do you think is the most valuable to an organization? 2. How do you critically associate the ideas of business agility and business efficiency...
-
Write a Verilog description of the following combinational circuit using concurrent statements. Each gate has a 5-ns delay, excluding the inverter, which has a 2-ns delay. C - ABCDAR
-
(a) Write Verilog code for a full subtracter using logic equations. (b) Write Verilog code for a 4-bit subtracter using the module defined in (a) as a component.
-
Write Verilog code for the following circuit. Assume that the gate delays arenegligible. (a) Using concurrent statements. (b) Using an always block with sequential statements. No latches should be...
-
A firecracker explodes in midair. Considering all the fragments upon explosion: total KE remains constant total momentum decreases total KE decreases total momentum is constant none of these (a) (b)...
-
On December 31, 2022, Cruise Company has 15,000 units of an inventory item, which costs $45 per unit when purchased on June 15, 2022. The selling price was $80 per unit. On December 30, 2022, it was...
-
1. The following inventory transactions took place for Bowman Corporation for the month of March 2022. Bowman uses a perpetual inventory system. Date Transaction Quantity Unit Cost / Selling Price...

Study smarter with the SolutionInn App


