Water can be decomposed to its elements, H 2 and O 2 , using electrical energy or
Question:
Water can be decomposed to its elements, H2 and O2, using electrical energy or in a series of chemical reactions. The following sequence of reactions is one possibility: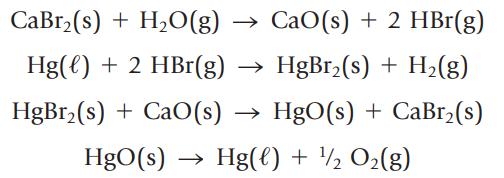
(a) Show that the net result of this series of reactions is the decomposition of water to its elements.
(b) If you use 1000. kg of water, what mass of H2 can be produced?
(c) Calculate the value of ∆rH° for each step in the series. Are the reactions predicted to be exo- or endothermic? ![AfH [CaBr(s)] = -683.2 kJ/mol AH [HgBr(s)] = -169.5 kJ/mol](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1697/2/6/9/555652a4733a39f71697269555636.jpg)
(d) Comment on the commercial feasibility of using this series of reactions to produce H2(g) from water.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





