Wet limestone is used to scrub SO 2 gas from the exhaust gases of power plants. One
Question:
Wet limestone is used to scrub SO2 gas from the exhaust gases of power plants. One possible reaction gives hydrated calcium sulfite:![]()
Another reaction gives hydrated calcium sulfate:![]()
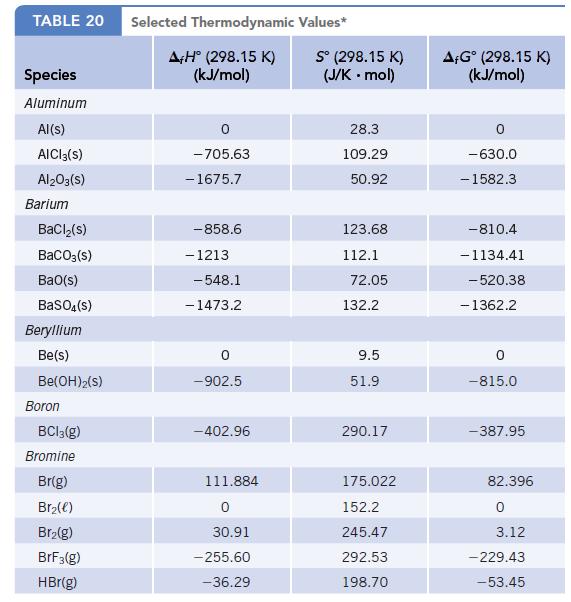
(a) Which reaction is more product-favored at equilibrium? Use the data in the table below and any other information needed in Appendix L to calculate ΔrG° for each reaction at 25°C.
(b) Calculate ΔrG° for the reaction CaSO3 ∙ 1⁄2 H2O(s) + 1⁄2 O2(g) ⇄ CaSO4 ∙ 1⁄2 H2O(s). Is this reaction product- or reactant-favored at equilibrium?
Data given in Appendix L
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





