Yeast can produce ethanol by the fermentation of glucose (C 6 H 12 O 6 ), which
Question:
Yeast can produce ethanol by the fermentation of glucose (C6H12O6), which is the basis for the production of most alcoholic beverages.
C6H12O6(aq) → 2 C2H5OH(ℓ) + 2 CO2(g)
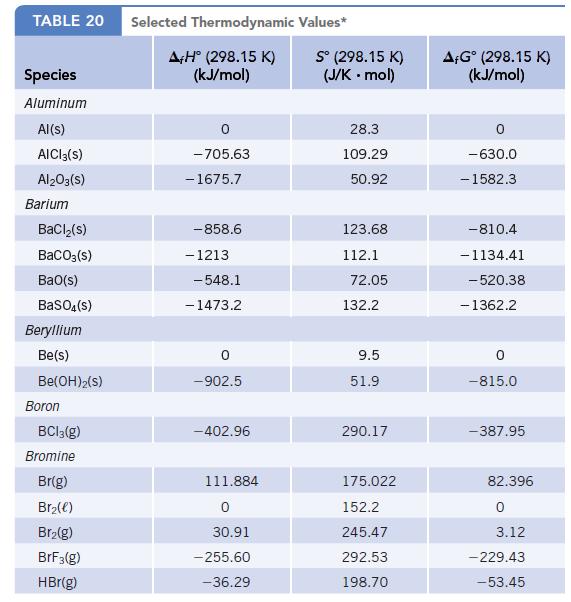
Calculate Δr H°, Δr S°, and ΔrG° for the reaction at 25°C. Is the reaction product- or reactant-favored at equilibrium? In addition to the thermodynamic values in Appendix L, you will need the following data for C6H12O6(aq):
Δf H° = −1260.0 kJ/mol; S° = 289 J/K ∙ mol; and Δf G° = −918.8 kJ/mol.
Data given in Appendix L
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





