You set out to determine the density of lead in the laboratory. Using a top loading balance
Question:
You set out to determine the density of lead in the laboratory. Using a top loading balance to determine the mass and the water displacement method (Study Question 41) to determine the volume of a variety of pieces of lead, you calculate the following densities: 11.6 g/cm3, 11.8 g/cm3, 11.5 g/cm3, and 12.0 g/cm3. You consult a reference book and find that the accepted value for the density of lead is 11.3 g/cm3. Calculate your average value, percent error, and standard deviation of your results.
Data given in Question 41
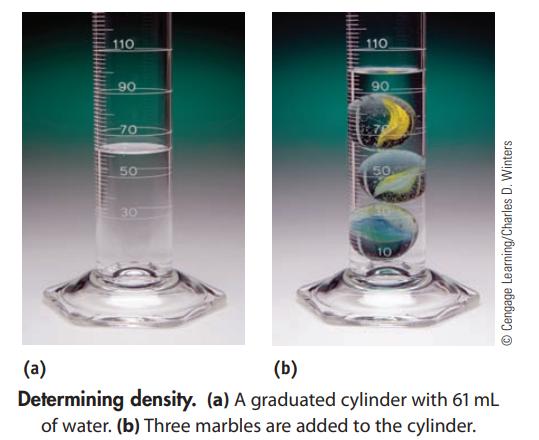
You have a 250.0-mL graduated cylinder containing some water. You drop three marbles with a total mass of 95.2 g into the water. What is the average density of a marble?
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel





