You wish to know the enthalpy change for the formation of liquid PCl 3 from the elements.
Question:
You wish to know the enthalpy change for the formation of liquid PCl3 from the elements. ![]()
The enthalpy change for the formation of PCl5 from the elements can be determined experimentally, as can the enthalpy change for the reaction of PCl3(ℓ) with more chlorine to give PCl5(s): 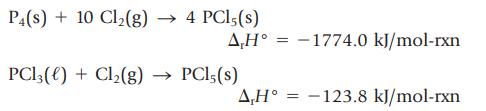
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





