A metallic sample is known to be barium, cesium, lithium, or silver. The electron binding energies for
Question:
A metallic sample is known to be barium, cesium, lithium, or silver. The electron binding energies for these metals are listed in the following table:
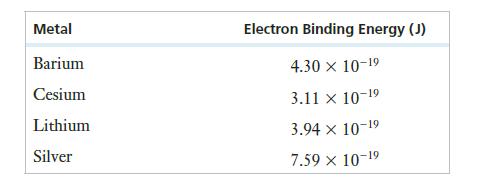
One way to identify the element might be through a photoelectric effect experiment. The experiment was performed three times, each time using a different laser as the light source. The results are summarized below. (The kinetic energy of the ejected photoelectrons was not measured.)
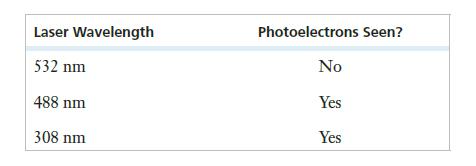
Based on this information, what conclusions can be drawn as to the identity of the metal?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





