Calculate the pressure of 15.0 g of methane gas in a 1.50-L vessel at 45.0C using: (a)
Question:
Calculate the pressure of 15.0 g of methane gas in a 1.50-L vessel at 45.0°C using:
(a) The ideal gas law and
(b) The van der Waals equation using constants in Table 5.2.
Table 5.2
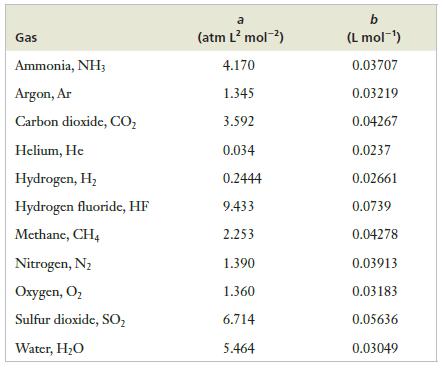
Transcribed Image Text:
Gas Ammonia, NH3 Argon, Ar Carbon dioxide, CO₂ Helium, He Hydrogen, H₂ Hydrogen fluoride, HF Methane, CH4 Nitrogen, N₂ Oxygen, O₂ Sulfur dioxide, SO₂ Water, H₂O a (atm L² mol-²) 4.170 1.345 3.592 0.034 0.2444 9.433 2.253 1.390 1.360 6.714 5.464 b (L mol-¹) 0.03707 0.03219 0.04267 0.0237 0.02661 0.0739 0.04278 0.03913 0.03183 0.05636 0.03049
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Calculate the pressure of 150 g of methane gas in a 150liter vessel at 450C usin...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
A system is described by x = Ax + Bu where and x1(0) = x2(0) = 10. Determine x1(t) and x2(t). 0 2 2
-
Kelly Lee, a junior audit staff, is assisting you being her audit manager in planning for the audit engagement. In a conversation between Kelly and you, she commented on the following, "In order to...
-
Find a least-squares solution of Ax = b by (a) Constructing the normal equations for x (b) Solving for x. A = 1-2 2 3 5 -1 0 2 b || 1 -4 2
-
Explain what you would assume the yield curve would look like during economic expansion and why.
-
What is synergy? What are some factors that might lead to synergy? How is the amount of synergy in a proposed merger measured, and how is it allocated between the two firms stockholder? Would the...
-
Mary Decker is suing the manufacturer of her car because of a defect that she believes caused her to have an accident, and kept her out of work for a year. She is suing the company for $3.5 million....
-
ZeeZee's Construction Company has the opportunity to select one of four projects (A, B, C, or D) or choose the null (do-nothing) alternative. Each project requires a single initial investment and has...
-
Margaret Black's family owns five parcels of farmland broken into a southeast sector, north sector, northwest sector, west sector, and southwest sector. Margaret is involved primarily in growing...
-
Path for Ampere's law Plane surface Bulging surface A parallel-plate capacitor with plates of radius R is being charged by the current ic. Recall Ampere's Law B.d-po Although there is no current...
-
A cylinder is filled with toxic COS gas to a pressure of 800.0 torr at 24C. According to the manufacturers specifications, the cylinder may rupture if the pressure exceeds 35 psi (pounds per square...
-
A cylinder containing 15.0 L of helium gas at a pressure of 165 atm is to be used to fill party balloons. Each balloon must be filled to a volume of 2.0 L at a pressure of 1.1 atm. What is the...
-
"Internal control can help organizations achieve efficiency of operations." Do you agree? Explain
-
Consider the following data on the number of hours that students slept: 5 6 6 7 7 7 8 8 8 8 8 9 9 9 9 9 9 1 0 1 1 1 1 what is the frequency of 5 - 6 , 7 - 8 , 9 - 1 0 , 1 1 - 1 2 ?
-
A block of mass m slides on a rough surface and moves toward a spring with a spring constant k = 3474 N/m, as shown in the figure below. When the block is d 19, 1 m away from the 15,7 m/s. When the...
-
Sunshine is now operating a plant that has the ability to generate a cash flow of $3 million per year. A new legislation that will be passed shortly can change the operating of this plant. After the...
-
need help in writing an email to my employees describing why I think "What if a female CEO acted like Elon Musk" would be a good read for them and why is it a valuable read?
-
Josh is going to draw a net of a triangular prism. How many rectangles should there be in his drawing?
-
Refer to the situation described in the previous exercise. Required: How might your solution differ if National Distribution Center prepares its financial statements according to International...
-
Find the reduced echelon form of each of the matrices given in Problems 120. c 1 26 + 4
-
The C 4v group has the following classes: E, 2C 4 , C 2 , 2 v , and 2 d . How many irreducible representations does this group have and what is the dimensionality of each? d refers to a dihedral...
-
Use the 2 Ã 2 matrices of Equation (27.10) to derive the multiplication table for the C 3v group. -(: ) ) - (: ) -sin 0 -cos 0 cos 0 -sin 0 1 1/2 V3/2 (V3/2 -1/2, -cos(27/3) -sin(27/3)...
-
Why can the signal loss resulting from spin dephasing caused by magnetic field in-homogeneities and chemical shift be recovered in the spin-echo experiment?
-
East Bank's lending provisions (for bad and doubtful debt) in its balance sheet as at the 1/1/2020 is $87.6 million. East Bank predicts that $36.8 million of its existing pool of loans will be...
-
How do interdisciplinary approaches, such as ecological economics, institutional economics, and behavioral finance, enrich our understanding of complex socio-economic systems by integrating insights...
-
The power requirement of an electronic portable device is 1.5Amp at 12 volts. What is the minimum number of AA-size batteries required for powering this device? Assume one AA battery is capable of...

Study smarter with the SolutionInn App


