Consider the following thermodynamic data for oxides of manganese. (a) What is the correct chemical nomenclature for
Question:
Consider the following thermodynamic data for oxides of manganese.
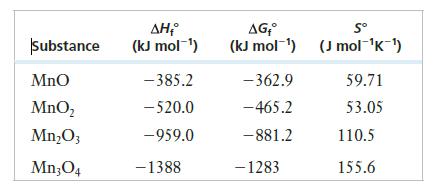
(a) What is the correct chemical nomenclature for each of the first three oxides?
(b) Write and balance chemical equations for the conversion of each of these oxides into Mn3O4.
(c) Based on the free energy changes of these reactions, which oxide is the most stable at room temperature?
Transcribed Image Text:
Substance Mno MnO₂ Mn₂O3 Mn304 ΔΗ (kJ mol-¹) -385.2 - 520.0 -959.0 - 1388 AG₁° Sº (kJ mol-¹) (J mol-¹K-¹) -362.9 - 465.2 -881.2 - 1283 59.71 53.05 110.5 155.6
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a MnO manganeseII oxide MnO 2 manganeseIV oxide Mn 2 O 3 mangane...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Case Study: Quick Fix Dental Practice Technology requirements Application must be built using Visual Studio 2019 or Visual Studio 2017, professional or enterprise. The community edition is not...
-
Reid Corporation's balance sheet at January 1, 20X9 reflected the following balances: Cash & Receivables $ 30,000 Inventory $ 75,000 Land $125,00 Building & Equipment (net) $850,000 Common Stock...
-
The Webster Store shows the following information relating to one of its products. Inventory, January 1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . 300 units @ $17.50 Sales, January 8 . ....
-
1. Fill in the blanks in the following table. Note that the ordering of the first column has been scrambled. 2. When _____ (supply/demand) changes, the equilibrium price and the equilibrium quantity...
-
The following data are for Marvin Department Store. The account balances (in thousands) are for 2017. 1. Compute (a) the cost of goods purchased and (b) the cost of goods sold. 2. Prepare the income...
-
Green Golf Accessories sells golf shoes, gloves, and a laser-guided range-finder that measures distance. Shown below are unit cost and sales data. Fixed costs are $620,000. Instructions (a) Compute...
-
Change Janets Schedule In Question 5 Above, You Calculated What The Schedule Would Look Like If Janet Had Made One $285.05 Payment In January 2022. You Did It By Hand, But The Bankrate Calculator Has...
-
(a) When a chemical bond forms, what happens to the entropy of the system? (b) Thermodynamically, what allows any bond formation to occur? (c) What do your answers to parts (a) and (b) suggest must...
-
Ammonia can react with oxygen gas to form nitrogen dioxide and water. (a) Write a balanced chemical equation for this reaction. (b) Use tabulated data to determine the free energy change for the...
-
What effects might the devaluation of a nations currency have on its business firms? On its consumers? On the debts it owes to other nations?
-
How are biological phenomena such as transcription and translation, alongside evolutionary mechanisms like natural selection, leveraged to enhance the quality of life for individuals globally?
-
All Sports Ltd supplies a wide range of sporting goods including Rugby League Balls. All Sports Ltd derives Australian sourced income for the current tax year comprising net income from trading of...
-
A machine with a cost of $52,000 has an estimated residual value of $4,246 and an estimated life of 5 years or 16,907 hours. What is the amount of depreciation for the second full year, using the...
-
What format allows students to easily share their portfolio with potential employers and is being required by a growing number of teacher preparation programs?
-
During March 2019, Annapolis Corporation recorded $42,800 of costs related to factory overhead. Alpha's overhead application rate is based on direct labor hours. The preset formula for overhead...
-
The following is a December 31, 2011, post-closing trial balance for the Vosburgh Electronics Corporation. Additional information: 1. The common stock represents 1 million shares of no par stock...
-
During registration at Tech every quarter, students in the Department of Management must have their courses approved by the departmental advisor. It takes the advisor an average of 4 minutes...
-
Consider the first-order correction to the energy of interacting spins illustrated in Example Problem 28.3 for 2 . Calculate the energy correction to the wave functions 1 = (1) (2), 2 = (1) (2),...
-
What is the difference between a configuration and a permutation?
-
What are the elements of a probability model, and how do they differ for continuous and discrete variables?
-
Two fixed charges, QA and QB, are located along the x-axis, as shown. Q = +4 10-8 C 5 cm QB = -2x 10-8 C 10 cm a) Find the net electric field (magnitude and direction) at the point labeled x. b) What...
-
A charge Q = +3 C is located at (-5 cm, 0) while charge Q = -4 C is located at (0, +5 cm). These are the two white charges in the diagram at right. a. What is the electric field at the origin (0, 0)...
-
Complete the table below. Data Table 6.1. Data and results of computations Height y, "Falling time t, s Average of falling time t, Square of falling time 12 Velocity Height y=. v = gt, 2g 772 m/s %...

Study smarter with the SolutionInn App


