Discuss the effect of temperature change on the spontaneity of the following reactions at 1 atm. (a)
Question:
Discuss the effect of temperature change on the spontaneity of the following reactions at 1 atm.
(a) 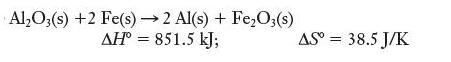
(b) 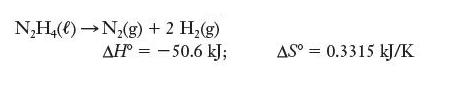
(c) 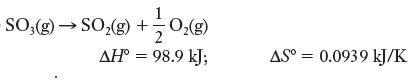
Transcribed Image Text:
Al₂O3(s) +2 Fe(s)→2 Al(s) + Fe₂O3(s) ΔΗ° = 851.5 kJ; AS = 38.5 J/K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To determine the effect of temperature change on the spontaneity of the given reactions we can use t...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Consider the following lattice structure of generalization and specialization A X B Y C
-
Determine whether the quadrilateral is a trapezoid. Explain. A D B J C
-
Discuss the effect of temperature change on the spontaneity of the following reactions at 1 atm. (a) Al2O3(s) + 2Fe(s) > 2Al(s) + Fe2O3(s) [ H = +851.5 kJ; S = +38.5 J/K] (b) N2H4(l) > N2(g) +...
-
Someone offered the investment options to Hendry on January 1, 2023: 1. Hendry has to save up to 5 times the initial deposit of US $ 150,000/year. 2. The savings cannot be taken for 20 years until...
-
What is an asset retirement obligation? What is the proper accounting for an asset retirement obligation?
-
Using 1913 as the base year with a value of 100, the ENR construction cost index (CCI) for August 2009 was 8563.35. For August 2010, the CCI value was 8837.38. (a) What was the inflation rate for...
-
John and Jennifer Margeson entered into a contract to sell a weight-loss franchise business called Inches-A-Weigh to Theresa Artis. The parties memorialized their agreement in an Asset Purchase...
-
John, Jake, and Joe are partners with capital accounts of $90,000, $78,000, and $64,000 respectively. They share profits and losses in the ratio of 30:40:30. When the partners decide to liquidate,...
-
Shelby Woods owns and operates an ice cream factory, the finest of its kind in all the world. Given that pints of ice cream are meant to be identical (for the same flavor), her company uses process...
-
The combustion of acetylene was used in welders torches for many years because it produces a very hot flame: (a) Use data in Appendix E to calculate S for this reaction. (b) Calculate G and show that...
-
There is another free energy state function, the Helmholtz free energy (A), defined as A = E TS. Comparing this to the definition of G, we see that internal energy has replaced enthalpy in the...
-
Repeat Prob. 5 - 93 for a power dissipation of 4 kW.
-
Does the possession of a core competence guarantee success? If yes, explain why. If no, explain why.?
-
Briefly describe the connection between Income Statement and Balance Sheet.
-
Calculate break-even volume in units (rounded): Fixed costs $125,000 Price per unit 5.75 Variable costs per unit 3.75
-
CSU Corporation began operations on January 1, 2017. The following information is available for CSU on December 31, 2017: Accounts receivable 1,800 Retained earnings ? Supplies expense 200 Accounts...
-
Consider the following code fragment. What do we need to enforce on function myFun() so that classes BBB and CCC can be used polymorphically? class AAA {my Fun() {}; }; class BBB: public AAA {...};...
-
What effect do share dividends or share splits have on the computation of the weighted-average number of shares outstanding?
-
Charles owns an office building and land that are used in his trade or business. The office building and land were acquired in 1978 for $800,000 and $100,000, respectively. During the current year,...
-
What observable is used to measure the viscosity of a gas or liquid?
-
In describing viscosity, what system quantity was transported? What is the expression for viscosity in terms of particle parameters derived from gas kinetic theory?
-
At 30 km above the Earths surface (roughly in the middle of the stratosphere), the pressure is roughly 0.013 atm and the gas density is 3.74 Ã 1023 molecules/m3. Assuming N2 is representative...
-
Problem 4.67 A binary transmission system transmits a signal X (1 to send a 0 bit; +1 to send a "1" bit). The received signal is Y = XN where noise N has a zero-mean Gaussian distribution with...
-
To purchase a $1,300,000 house, you financed it with a 60% Loan to Value (LTV) ratio constant payment loan maturing in 25 years. The loan is fully amortising. The annual interest rate is 3.15% and...
-
Since WDCC's class size is 16 rather than 24 under Nursery Act. We assume 16 to conservatively calculate the payback period and ROI. Assumptions Area of Gymnasium (Square feet) 704 unobstructed space...

Study smarter with the SolutionInn App


