Experiments show that the reaction of nitrogen dioxide with fluorine, The reaction is thought to occur in
Question:
Experiments show that the reaction of nitrogen dioxide with fluorine,
![2 NO(g) + F(g) 2 FNO(g) has the rate law Rate = k[NO][F]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/0/7/4/96965645819936b91701074968278.jpg)
The reaction is thought to occur in two steps.
(a) Show that the sum of this sequence of reactions gives the balanced equation for the overall reaction.
(b) Which step is rate determining? 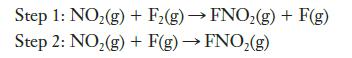
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





