Heating a 24.0-g aluminum can raises its temperature by 15.08C. Find the value of q for the
Question:
Heating a 24.0-g aluminum can raises its temperature by 15.08C. Find the value of q for the can.
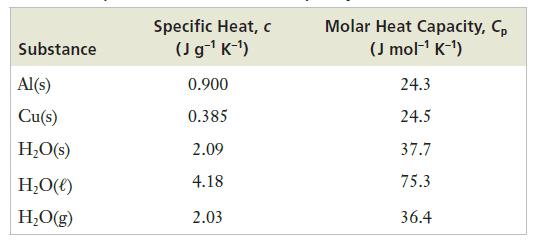
Strategy Heat flow depends on the heat capacity of the material being heated, the size of the sample, and the change in temperature. Because the information given is in mass, it will be easier to use the specific heat rather than the molar heat capacity.
The value of the specific heat of aluminum can be found in Table 9.2. Because the temperature change is given in 8C, we will write the units as J g−1 8C−1.
Table 9.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





